The balanced reaction of calcium carbonate and hydrochloric acid is as follows: CACO3 (s) + 2 HC1(aq) → CaCl2 (aq) + H2O (1)+ CO2 (g) Reaction equation of calcium carbonate and hydrochloric acid producing calcium chloride, water and carbon dioxide as products Record your moles and percent error calculations for calcium carbonate Trial 1 Trial 2 Trial 3 Value of CaCO3 reported on the bottle (mg) 500 Mass of antacid tablet used (g) 1.22 1.21 1.22 Moles of HCl from part I. .015000 .015000 .015000 Unrounded Moles of NaOH from part II. .0038610 .0039000 .0037080 Unrounded Moles of HCl neutralized by NaOH .011139 Unrounded Moles of HCl neutralized by NaOH Rounded Moles of HCl neutralized by CaCOз Unrounded Moles of HCl neutralized by CaCOз Rounded Moles of CaCO3 Unrounded Moles of CaCO03 Rounded Mass of CaCO3 in tablet (mg) Unrounded Mass of CACO3 in tablet (mg) Rounded Percent error (%, use rounded values) Unrounded Percent error (%) Rounded Percent effectiveness of tablet (%) Unrounded Percent effectiveness of tablet (%) Rounded
The balanced reaction of calcium carbonate and hydrochloric acid is as follows: CACO3 (s) + 2 HC1(aq) → CaCl2 (aq) + H2O (1)+ CO2 (g) Reaction equation of calcium carbonate and hydrochloric acid producing calcium chloride, water and carbon dioxide as products Record your moles and percent error calculations for calcium carbonate Trial 1 Trial 2 Trial 3 Value of CaCO3 reported on the bottle (mg) 500 Mass of antacid tablet used (g) 1.22 1.21 1.22 Moles of HCl from part I. .015000 .015000 .015000 Unrounded Moles of NaOH from part II. .0038610 .0039000 .0037080 Unrounded Moles of HCl neutralized by NaOH .011139 Unrounded Moles of HCl neutralized by NaOH Rounded Moles of HCl neutralized by CaCOз Unrounded Moles of HCl neutralized by CaCOз Rounded Moles of CaCO3 Unrounded Moles of CaCO03 Rounded Mass of CaCO3 in tablet (mg) Unrounded Mass of CACO3 in tablet (mg) Rounded Percent error (%, use rounded values) Unrounded Percent error (%) Rounded Percent effectiveness of tablet (%) Unrounded Percent effectiveness of tablet (%) Rounded
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 50A
Related questions
Question
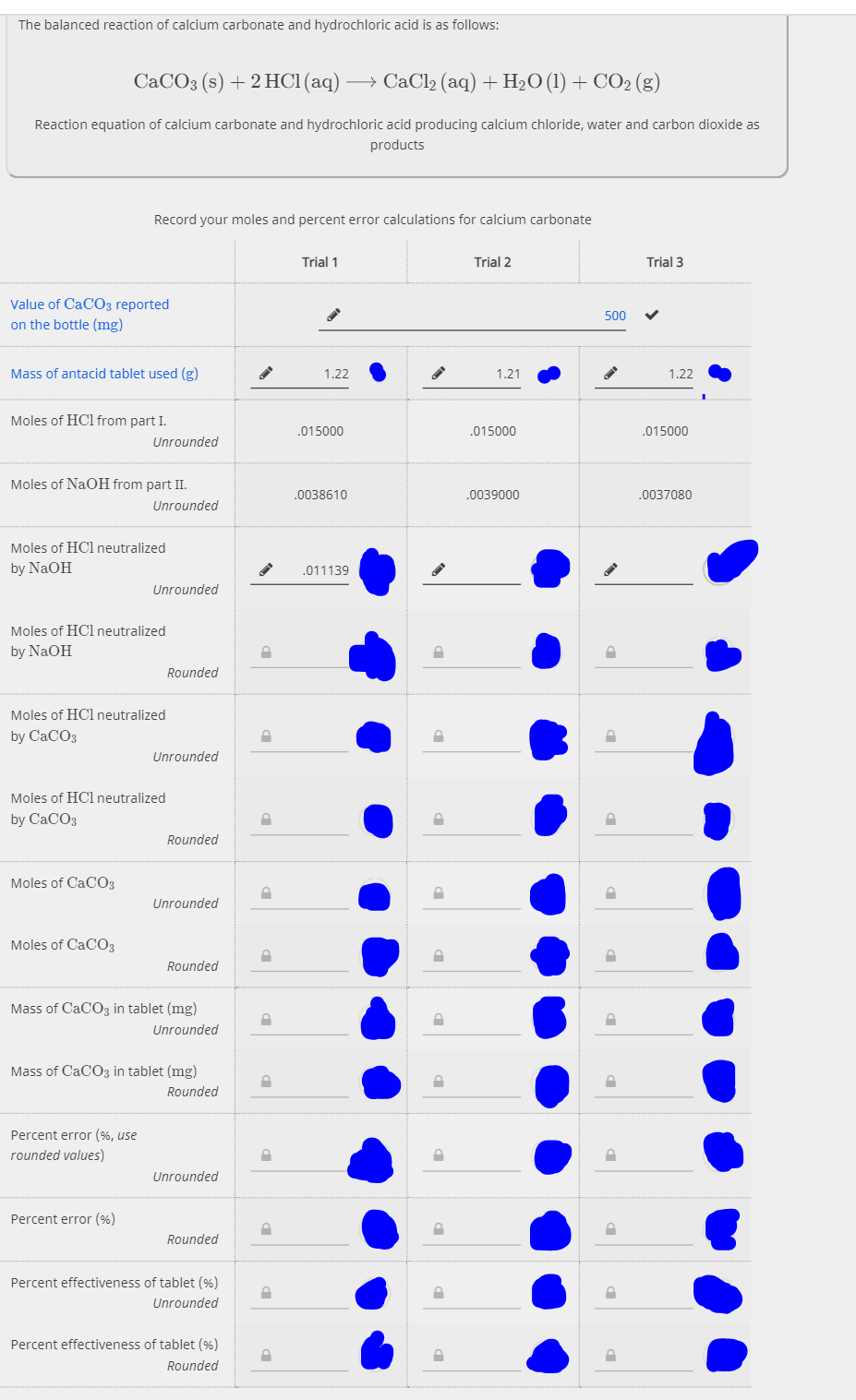
Transcribed Image Text:The balanced reaction of calcium carbonate and hydrochloric acid is as follows:
CACO3 (s) + 2 HC1(aq) → CaCl2 (aq) + H2O (1)+ CO2 (g)
Reaction equation of calcium carbonate and hydrochloric acid producing calcium chloride, water and carbon dioxide as
products
Record your moles and percent error calculations for calcium carbonate
Trial 1
Trial 2
Trial 3
Value of CaCO3 reported
500
on the bottle (mg)
Mass of antacid tablet used (g)
1.22
1.21
1.22
Moles of HCl from part I.
.015000
.015000
.015000
Unrounded
Moles of NaOH from part II.
.0038610
.0039000
.0037080
Unrounded
Moles of HCl neutralized
by NaOH
.011139
Unrounded
Moles of HCl neutralized
by NaOH
Rounded
Moles of HCl neutralized
by CaCОз
Unrounded
Moles of HCl neutralized
by CaCOз
Rounded
Moles of CaCO3
Unrounded
Moles of CaCO3
Rounded
Mass of CaCO3 in tablet (mg)
Unrounded
Mass of CaCO3 in tablet (mg)
Rounded
Percent error (%, use
rounded values)
Unrounded
Percent error (%)
Rounded
Percent effectiveness of tablet (%)
Unrounded
Percent effectiveness of tablet (%)
Rounded
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning