The buffer solution used in Experiment 2 was prepared by using stock solutions of a weak acid and its conjugate base. A buffer could also have been created if we added a strong base to a weak acid. How many milliters of 2.5 M NaOH should be added to 0.05 mol acetic acid to give a solution with a pH of 4.76 for a final volume of 500 mL? Give your answer to two decimal places.
The buffer solution used in Experiment 2 was prepared by using stock solutions of a weak acid and its conjugate base. A buffer could also have been created if we added a strong base to a weak acid. How many milliters of 2.5 M NaOH should be added to 0.05 mol acetic acid to give a solution with a pH of 4.76 for a final volume of 500 mL? Give your answer to two decimal places.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 54QAP: Ammonia gas is bubbled into 275 mL of water to make an aqueous solution of ammonia. To prepare a...
Related questions
Question
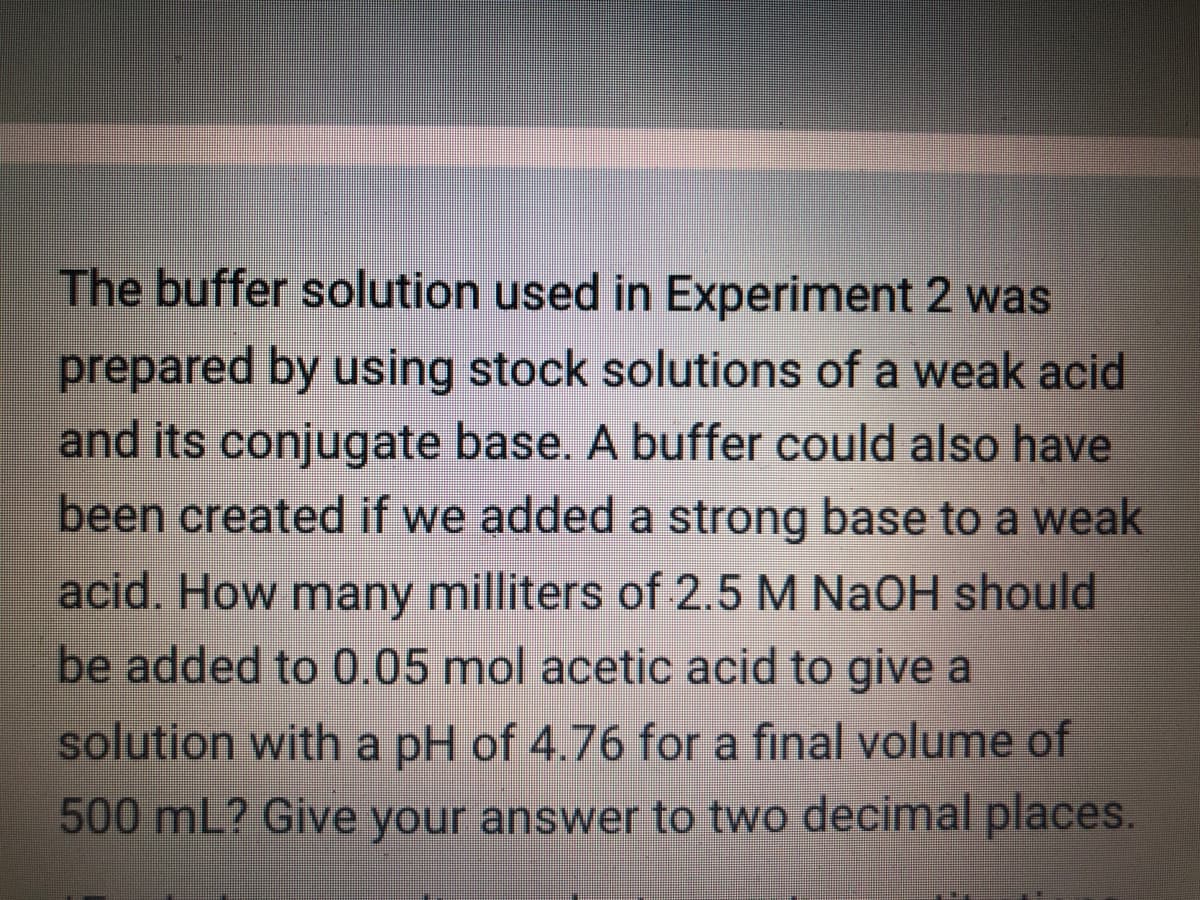
Transcribed Image Text:The buffer solution used in Experiment 2 was
prepared by using stock solutions of a weak acid
and its conjugate base. A buffer could also have
been created if we added a strong base to a weak
acid. How many milliters of 2.5 M NaOH should
be added to 0.05 mol acetic acid to give a
solution with a pH of 4.76 for a final volume of
500 mL? Give your answer to two decimal places.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning