The column was packed with 12 g of alumina. A 50:50 mixture of ferrocene and acetylferrocene was weighed on the electronic balance (total mass = 0.102 g, or 102 mg), and placed at the top of the column. Elution was first with hexane, which caused a yellow band to move down the white alumina. After the yellow fraction was collected, the eluent was replaced by TBME, which was passed through the column until the red fraction was collected. The two colored solutions were heated in a 70 °C water bath until the solvents were evaporated and colored solids were obtained. Percent recoveries for each compound were calculated, and melting points were taken. Data for the first, fastest-moving fraction: 0.045 g yellow solid, mp = 170-171 °C. Data for the second, slowest-moving fraction: 0.054 g orange solid, mp= 75-80 °C.
The column was packed with 12 g of alumina. A 50:50 mixture of ferrocene and acetylferrocene was weighed on the electronic balance (total mass = 0.102 g, or 102 mg), and placed at the top of the column. Elution was first with hexane, which caused a yellow band to move down the white alumina. After the yellow fraction was collected, the eluent was replaced by TBME, which was passed through the column until the red fraction was collected. The two colored solutions were heated in a 70 °C water bath until the solvents were evaporated and colored solids were obtained. Percent recoveries for each compound were calculated, and melting points were taken. Data for the first, fastest-moving fraction: 0.045 g yellow solid, mp = 170-171 °C. Data for the second, slowest-moving fraction: 0.054 g orange solid, mp= 75-80 °C.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.8QAP
Related questions
Question
calculate for percent recovery, and explain what does 50:50 mixture means? since total mass is 0.102 g, does it means 0.051 for ferrocene, and another 0.051 for acetyferrocene. I feel confused.
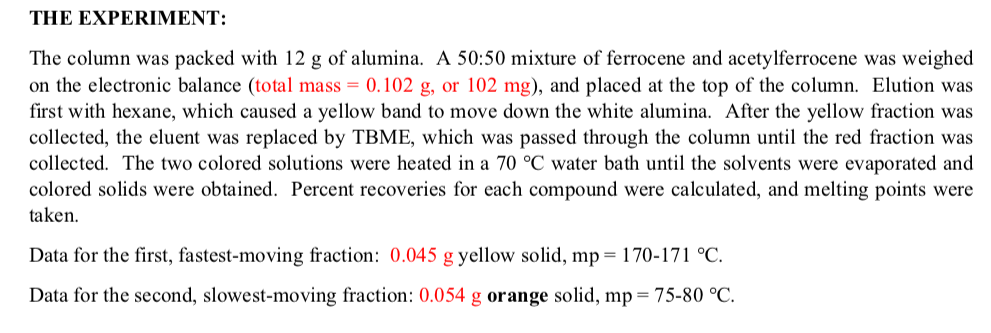
Transcribed Image Text:THE EXPERIMENT:
The column was packed with 12 g of alumina. A 50:50 mixture of ferrocene and acetylferrocene was weighed
on the electronic balance (total mass = 0.102 g, or 102 mg), and placed at the top of the column. Elution was
first with hexane, which caused a yellow band to move down the white alumina. After the yellow fraction was
collected, the eluent was replaced by TBME, which was passed through the column until the red fraction was
collected. The two colored solutions were heated in a 70 °C water bath until the solvents were evaporated and
colored solids were obtained. Percent recoveries for each compound were calculated, and melting points were
taken.
Data for the first, fastest-moving fraction: 0.045 g yellow solid, mp= 170-171 °C.
Data for the second, slowest-moving fraction: 0.054 g orange solid, mp = 75-80 °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT