The Standard free energy CAG0¹) of the reaction shown above can be estimated based on? A. High Energy bands B. Reduction potential C. cannot be estimated The reaction shown above requires the cofactor to proceed forward without significant was te OF A.ATP energy. B. Nicotinamide C. Flavin D. No cofactor required Determine approximate A6% of the coupled reaction KJ/mol possible answers: (0,-8,-15,-22,-30,−38,-45) The class of enzyme that catalyzes the reaction is possible answers: Mutase, Isomerase, ki nase, phosphatase, Dehydrogenase, Aldolase
The Standard free energy CAG0¹) of the reaction shown above can be estimated based on? A. High Energy bands B. Reduction potential C. cannot be estimated The reaction shown above requires the cofactor to proceed forward without significant was te OF A.ATP energy. B. Nicotinamide C. Flavin D. No cofactor required Determine approximate A6% of the coupled reaction KJ/mol possible answers: (0,-8,-15,-22,-30,−38,-45) The class of enzyme that catalyzes the reaction is possible answers: Mutase, Isomerase, ki nase, phosphatase, Dehydrogenase, Aldolase
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter20: Electron Transport And Oxidative Phosphorylation
Section: Chapter Questions
Problem 12P
Related questions
Question
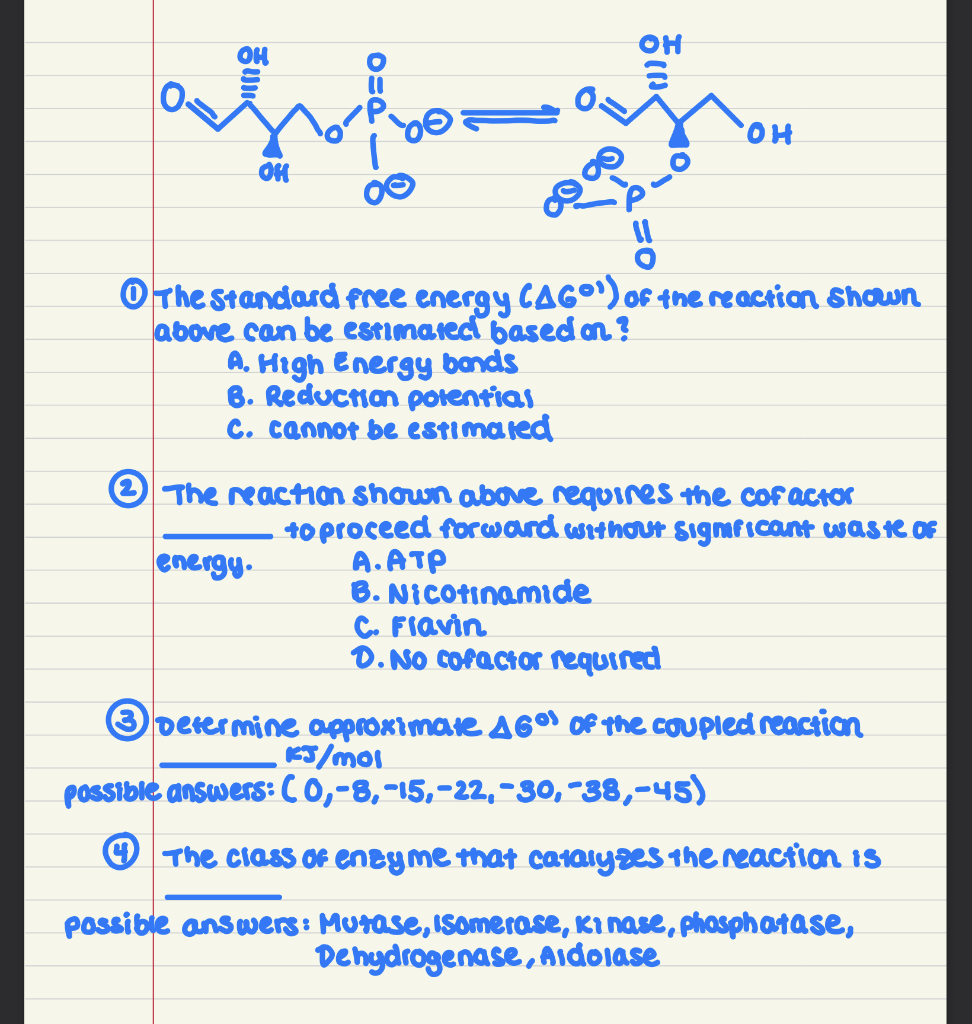
Transcribed Image Text:OH
affromah.
The Standard free energy CAGO¹) of the reaction shown
above can be estimated based on?
OH
A. High Energy bands
B. Reduction potential
C. cannot be estimated
OH
energy.
он
The reaction shown above requires the cofactor
to proceed forward without significant was te of
A.ATP
B. Nicotinamide
C. Flavin
D. No cofactor required
3 Determine approximate 46°1 of the coupled reaction
KJ/mol
possible answers: (0,-8,-15,-22,-30, -38,-45)
The class of enzyme that catalyzes the reaction is
possible answers: Mutase, Isomerase, ki nase, phosphatase,
Dehydrogenase, Aidolase
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning