To determine the molar concentration of a metal ion in a solution of unknown concentration, a student fırst made five standard solutions that contain the metal ion of interest and measured the absorbance of each solution in a spectrophotometer at its åmax- A calibration curve was obtained that had an equation of y = 5.747 x + 0.013 Next, the student pipetted 15.0 mL of the initial solution of unknown concentration into a 100.0 mL volumetric flask, and filled the flask with deionized water to the line. The absorbance of this final diluted solution was found to be A = 0.226 at Amax- The color of the original and diluted solution was blue. What is the molarity of the original solution, as well approximate Amax for this metal ion? Amax = 599 nm and concentration is 0.247 M %3D Amax = 457 nm and concentration is 0.247 M %3D i max = 457 nm and concentration is 0.0371 M a max = 599 nm and concentration is 0.0371 M %3D Amax = 599 nm and concentration is 0.00557 M %3D
To determine the molar concentration of a metal ion in a solution of unknown concentration, a student fırst made five standard solutions that contain the metal ion of interest and measured the absorbance of each solution in a spectrophotometer at its åmax- A calibration curve was obtained that had an equation of y = 5.747 x + 0.013 Next, the student pipetted 15.0 mL of the initial solution of unknown concentration into a 100.0 mL volumetric flask, and filled the flask with deionized water to the line. The absorbance of this final diluted solution was found to be A = 0.226 at Amax- The color of the original and diluted solution was blue. What is the molarity of the original solution, as well approximate Amax for this metal ion? Amax = 599 nm and concentration is 0.247 M %3D Amax = 457 nm and concentration is 0.247 M %3D i max = 457 nm and concentration is 0.0371 M a max = 599 nm and concentration is 0.0371 M %3D Amax = 599 nm and concentration is 0.00557 M %3D
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.10QAP: The acid-base indicator HIn undergoes the following reaction in dilute aqueous solution:...
Related questions
Question
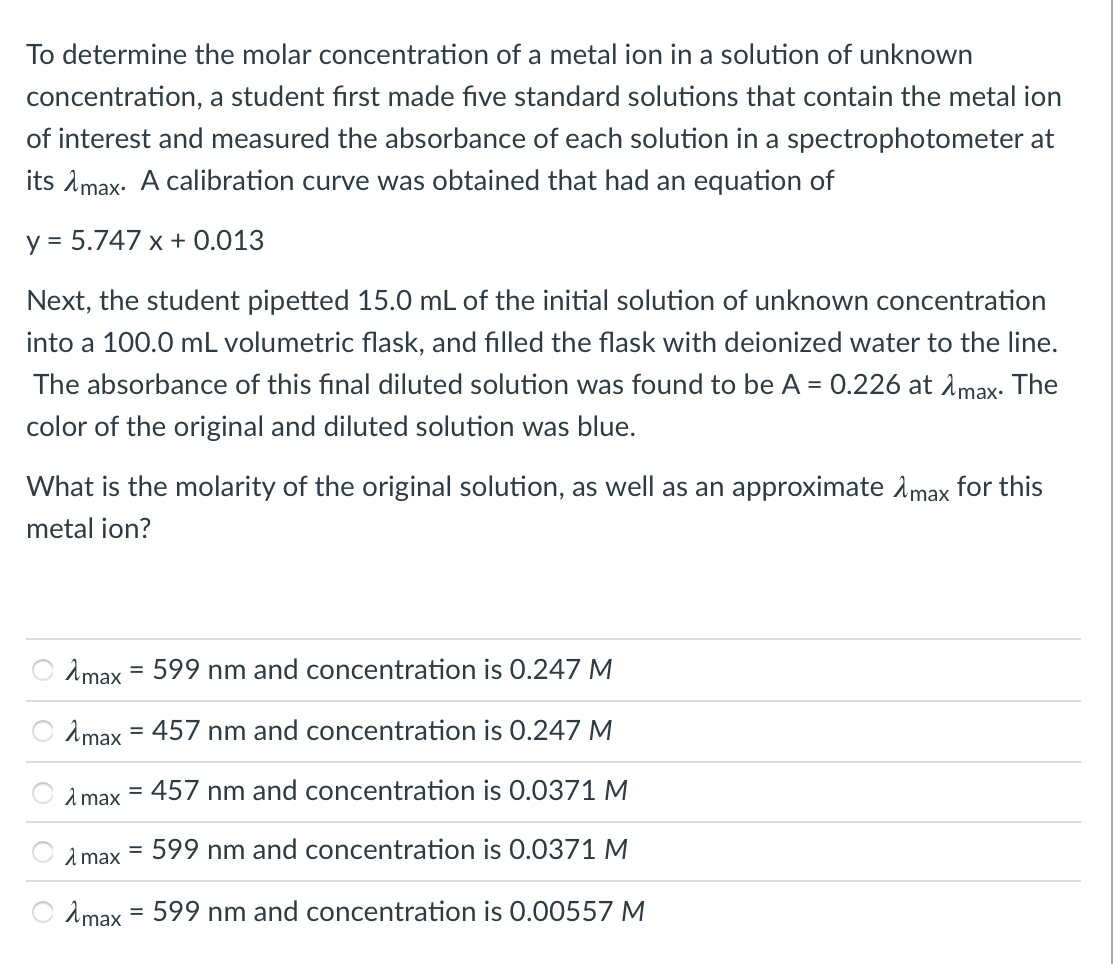
Transcribed Image Text:To determine the molar concentration of a metal ion in a solution of unknown
concentration, a student fırst made five standard solutions that contain the metal ion
of interest and measured the absorbance of each solution in a spectrophotometer at
its Amax- A calibration curve was obtained that had an equation of
y = 5.747 x + 0.013
Next, the student pipetted 15.0 mL of the initial solution of unknown concentration
into a 100.0 mL volumetric flask, and filled the flask with deionized water to the line.
The absorbance of this final diluted solution was found to be A = 0.226 at Amax. The
color of the original and diluted solution was blue.
What is the molarity of the original solution, as well as an approximate Amax for this
metal ion?
2max = 599 nm and concentration is 0.247 M
Amax
457 nm and concentration is 0.247 M
%3D
2 max
= 457 nm and concentration is 0.0371 M
1 max
599 nm and concentration is 0.0371 M
Amax = 599 nm and concentration is 0.00557 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
