TUI 14eaction Progress Lab Activity Name 2. Consider the following chemical reaction. 3Zn(s) + 2H3PO4(aq) → Zna(PO4)2(s) + 3H2(g) IT 15.0 grams of Zn are added to 250.0mL of 0.580M H3PO4 and allowed to react for To mindtes, 21.2 grams of Zn3(PO4)2(s) are formed, What concentration of H3PO4 remains after 10 minutes (assume total solution volume of 250.0 mL) 3H2(g) -> Zn3(PO4)2(s) 3Zn(s) 2H3PO4(aq) initial moles change in moles (in terms of x) new moles (expression) new moles (numerical value) Show calculations below:
TUI 14eaction Progress Lab Activity Name 2. Consider the following chemical reaction. 3Zn(s) + 2H3PO4(aq) → Zna(PO4)2(s) + 3H2(g) IT 15.0 grams of Zn are added to 250.0mL of 0.580M H3PO4 and allowed to react for To mindtes, 21.2 grams of Zn3(PO4)2(s) are formed, What concentration of H3PO4 remains after 10 minutes (assume total solution volume of 250.0 mL) 3H2(g) -> Zn3(PO4)2(s) 3Zn(s) 2H3PO4(aq) initial moles change in moles (in terms of x) new moles (expression) new moles (numerical value) Show calculations below:
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
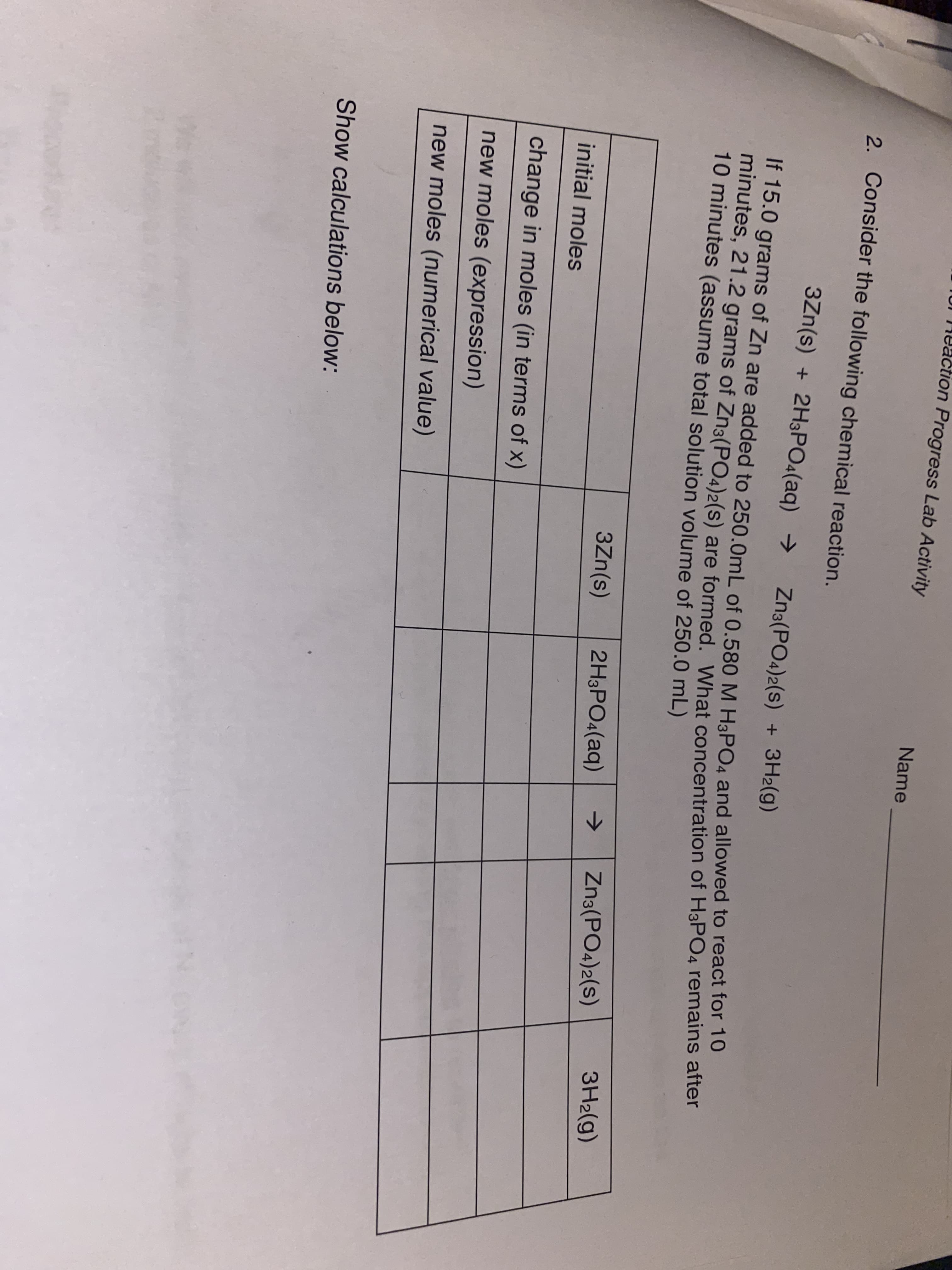
Transcribed Image Text:TUI 14eaction Progress Lab Activity
Name
2. Consider the following chemical reaction.
3Zn(s) + 2H3PO4(aq) →
Zna(PO4)2(s)
+ 3H2(g)
IT 15.0 grams of Zn are added to 250.0mL of 0.580M H3PO4 and allowed to react for To
mindtes, 21.2 grams of Zn3(PO4)2(s) are formed, What concentration of H3PO4 remains after
10 minutes (assume total solution volume of 250.0 mL)
3H2(g)
->
Zn3(PO4)2(s)
3Zn(s)
2H3PO4(aq)
initial moles
change in moles (in terms of x)
new moles (expression)
new moles (numerical value)
Show calculations below:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

