Uracil and Adenine are two nitrogen bases constitutive of RNA. Both compounds show maximum absorption spectra at 260 nm. A 20 µmol.L¹ uracil solution gives an absorbance value of 0.24 at 260 nm (in a 1 cm-optical path length cuvette). 1- Calculate the molar extinction coefficient of uracil at 260 nm. 2- A solution containing a mixture of unknown concentrations of adenine and uracil shows an absorbance value at 260 nm of 0.4 (1 cm-optical path length cuvette). Considering that the molar concentration of uracil is twice the one of adenine, and that the molar extinction coefficient of adenine at 260 nm is 16000 mol¹.L.cm-¹, calculate the molar concentrations of adenine and uracil in the mixture.
Uracil and Adenine are two nitrogen bases constitutive of RNA. Both compounds show maximum absorption spectra at 260 nm. A 20 µmol.L¹ uracil solution gives an absorbance value of 0.24 at 260 nm (in a 1 cm-optical path length cuvette). 1- Calculate the molar extinction coefficient of uracil at 260 nm. 2- A solution containing a mixture of unknown concentrations of adenine and uracil shows an absorbance value at 260 nm of 0.4 (1 cm-optical path length cuvette). Considering that the molar concentration of uracil is twice the one of adenine, and that the molar extinction coefficient of adenine at 260 nm is 16000 mol¹.L.cm-¹, calculate the molar concentrations of adenine and uracil in the mixture.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
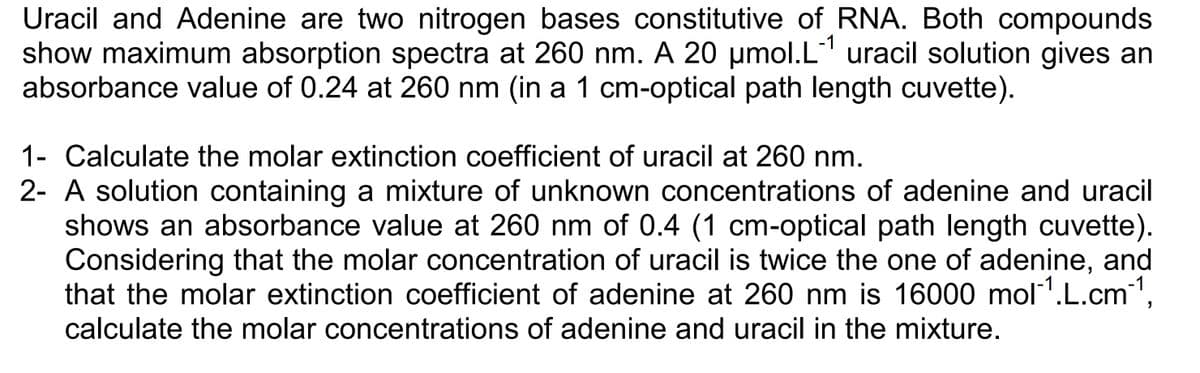
Transcribed Image Text:Uracil and Adenine are two nitrogen bases constitutive of RNA. Both compounds
show maximum absorption spectra at 260 nm. A 20 µmol.L-¹ uracil solution gives an
absorbance value of 0.24 at 260 nm (in a 1 cm-optical path length cuvette).
1- Calculate the molar extinction coefficient of uracil at 260 nm.
2- A solution containing a mixture of unknown concentrations of adenine and uracil
shows an absorbance value at 260 nm of 0.4 (1 cm-optical path length cuvette).
Considering that the molar concentration of uracil is twice the one of adenine, and
that the molar extinction coefficient of adenine at 260 nm is 16000 mol¹.L.cm-¹,
calculate the molar concentrations of adenine and uracil in the mixture.
-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON