
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
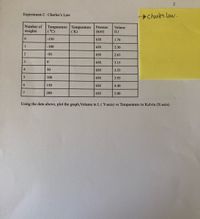
Transcribed Image Text:Charle's Law.
Experiment-2 : Charles's Law
Number of
Temperature Temperature
(°C)
Pressure
Volume
weights
(К)
(torr)
(L)
0.
-150
650
1.76
1
-100
650
2.30
-50
650
2.65
0.
650
3.15
4
50
650
3.55
100
650
3.95
6.
150
650
4.40
7
200
650
5.00
Using the data above, plot the graph,Volume in L( Y-axis) vs Temperature in Kelvin (X-axis)
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- part carrow_forwardA 0.28 g sample of carbon dioxide has a volume of 1.04 L and pressure of 1.09 atm. What is the temperature of the gas in K? R = 0.0821 Present the answer with one digit after the decimal point.arrow_forwardCovert 20.5 mL to mm^3 Convert 102 degrees Fahrenheit to Kelvin Answer in g/mL (54.38 g - 50.06 g)/ 2.35 mLarrow_forward
- Convert 58.5°F into celsius unitarrow_forwardIf a scuba diver is experiencing 5.0 atm. To what depth in feet has he dived? Given, the surface of the sea is 1.0 atm and density if Mercury is 13.6g/ml, the density of water is 1.00g/ml and there are 3.28 ft on one meter.arrow_forwardplease answer quicklyarrow_forward
- If the temperature for one of the runs in 52 degrees celcius, what is the 1/temperature(K)y value that will be plotted?arrow_forwardI was delivering a Star Wars Yoda helium balloon to my son’s birthday party. There was 10 liters of air in the ballon when it was filled indoors at 297K. Went outside, I did! Nine (9 )liters of air, there now is! What is outside temperature in (Kelvins)?arrow_forwardJ. A balloon filled with 1.22 L of gas at 286 K is heated until the volume is 2.86 L while the pressure remains constant. What is the temperature of the gas in Kelvin? K Your answer should be rounded to the nearest whole number. Do not include units in your answer. Enter the answer Check it skip - GET A HINT 2 SCRATCHPAD o Improve this question 近arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY