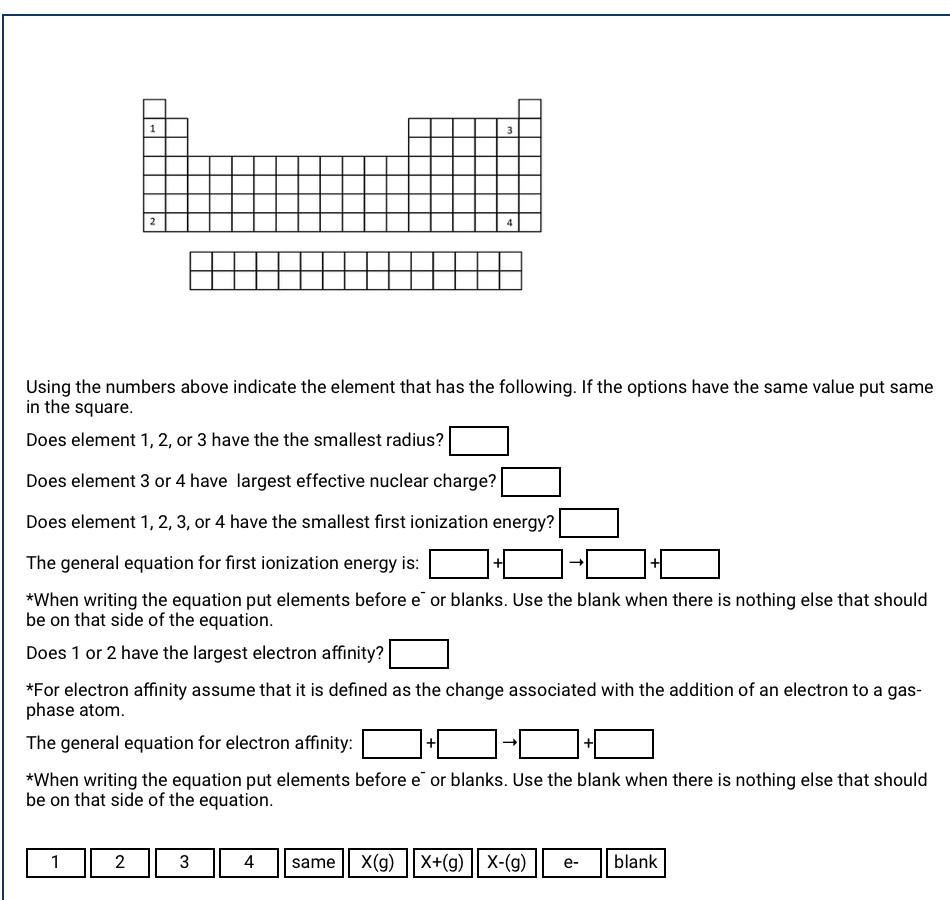
Using the numbers above indicate the element that has the following. If the options have the same value put same in the square. Does element 1, 2, or 3 have the the smallest radius? Does element 3 or 4 have largest effective nuclear charge? Does element 1, 2, 3, or 4 have the smallest first ionization energy?
Using the numbers above indicate the element that has the following. If the options have the same value put same in the square. Does element 1, 2, or 3 have the the smallest radius? Does element 3 or 4 have largest effective nuclear charge? Does element 1, 2, 3, or 4 have the smallest first ionization energy?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 46GQ: Name the element corresponding to each characteristic below. (a) the element with the electron...
Related questions
Question
Transcribed Image Text:2
Using the numbers above indicate the element that has the following. If the options have the same value put same
in the square.
Does element 1, 2, or 3 have the the smallest radius?
Does element 3 or 4 have largest effective nuclear charge?
Does element 1, 2, 3, or 4 have the smallest first ionization energy?
The general equation for first ionization energy is:
+
*When writing the equation put elements before e or blanks. Use the blank when there is nothing else that should
be on that side of the equation.
Does 1 or 2 have the largest electron affinity?
*For electron affinity assume that it is defined as the change associated with the addition of an electron to a gas-
phase atom.
The general equation for electron affinity:
*When writing the equation put elements before e or blanks. Use the blank when there is nothing else that should
be on that side of the equation.
1
3
4
X(9) | x+(9) || х-(9)
blank
same
e-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning