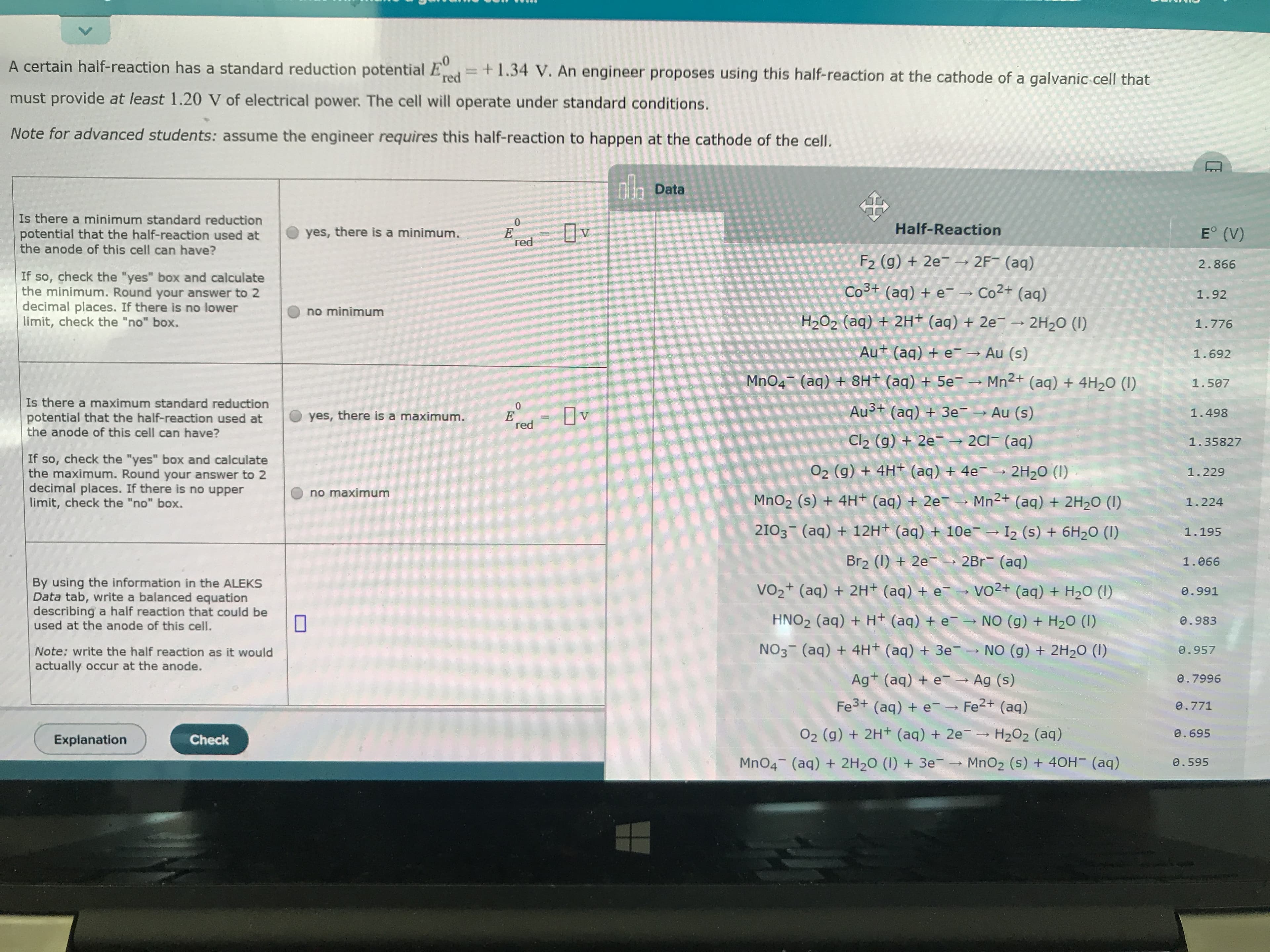
V 0 A certain half-reaction has a standard reduction potential E +1.34 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that red must provide at least 1.20 V of electrical power The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. nh Data Is there a minimum standard reduction 0 E red Half-Reaction E (V) yes, there is a minimum. potential that the half-reaction used at the anode of this cell can have? 2F (aq) F2 (g) + 2e 2.866 If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. Co3t (aq) + e Co2+ (aq) 1.92 no minimum -2H20 (I) H202 (aq) + 2Ht (aq) + 2e 1.776 Aut (aq) + e Au (s) 1.692 MnO4 (aq) + 8Ht (aq) + 5e Mn2 (aq) + 4H20 (I) 1.507 Is there a maximum standard reduction 0 E red Au3t (aq) +3e Au (s) 1.498 yes, there is a maximum. potential that the half-reaction used at the anode of this cell can have? Cl2 (g) + 2e 2Cl (aq) 1.35827 If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. O2 (g) + 4H (aq) + 4e 2H20 (I) 1.229 no maximum Mn2+ (aq) + 2H20 (I) MnO2 (s) +4H+ (aq) + 2e 1.224 2103 (aq) 12H (aq) + 10e I2 (s) + 6H20 (I) 1.195 Br2 (I)2e 2Br (aq) 1.066 By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the anode of this cell. vo2+ (aq) + H2O (I) VO2+ (aq) 2H+ (aq) + e 0.991 HNO2 (aq) + H+ (aq) + e NO (g) + H20 (I) 0.983 N3 (aq) 4H+ (aq) + 3e- NO (g)+ 2H20 (I) 0.957 Note: write the half reaction as it would actually occur at the anode. Ag (aq) e Ag (s) 0.7996 Fe3+ (aq) + e Fe2+ (aq) 0.771 O2 (g) 2H(aq) + 2e H2O2 (aq) 0.695 Explanation Check MnO4 (aq) + 2H20 (I) 3e MnO2 (s) + 40H- (aq) 0.595
Science behind corrosion-test
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Corrosion
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Is there a minimum or maximum standard reduction potential that the half-reaction can have. Then write the balanced equation
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images









