Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
In this double titration experiment determining composition of soda ash, you are given the following data:
- M HCl titrant: 0.0501 M +/- 0.0507
- Mass of soda ash sample, g: 0.2505 ± 2.0e-4 (Analytical Balance)
- Total volume of Sample Stock, mL: 75 ± 0.05 (100mL graduated cylinder)
- Volume of Sample Stock Aliquot, mL: 25 ± 0.3
Find the missing information. Show solutions
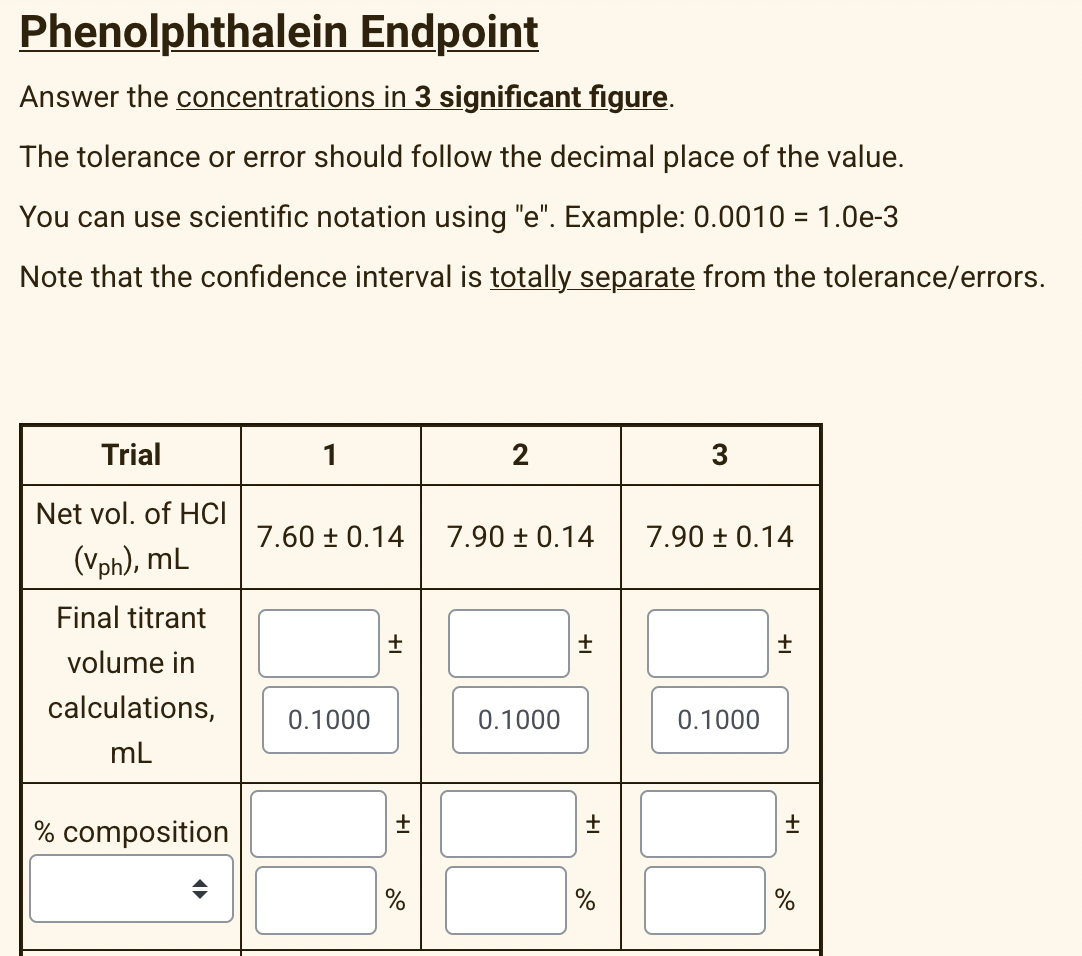
Transcribed Image Text:Phenolphthalein Endpoint
Answer the concentrations in 3 significant figure.
The tolerance or error should follow the decimal place of the value.
You can use scientific notation using "e". Example: 0.0010 = 1.0e-3
Note that the confidence interval is totally separate from the tolerance/errors.
Trial
1
2
Net vol. of HCI
7.60 ± 0.14
7.90 + 0.14
7.90 + 0.14
(Vph), ML
Final titrant
volume in
calculations,
0.1000
0.1000
0.1000
mL
% composition
+I
+1
+1
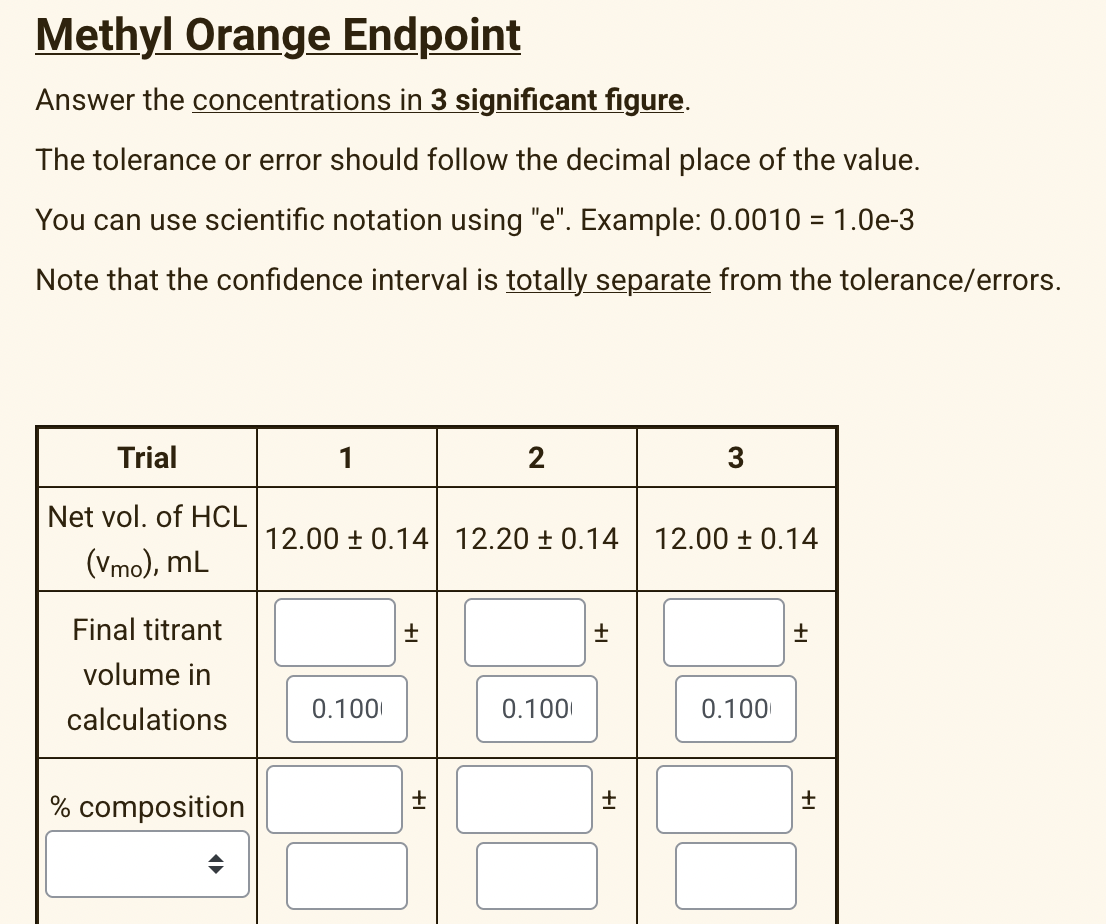
Transcribed Image Text:Methyl Orange Endpoint
Answer the concentrations in 3 significant figure.
The tolerance or error should follow the decimal place of the value.
You can use scientific notation using "e". Example: 0.0010 = 1.0e-3
Note that the confidence interval is totally separate from the tolerance/errors.
Trial
1
2
3
Net vol. of HCL
12.00 ± 0.14 12.20 ± 0.14
12.00 ± 0.14
(Vmo), mL
Final titrant
volume in
calculations
0.100
0.100
0.100
% composition
+1
+I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning