
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:view-source:https://...
* Question Completion Status:
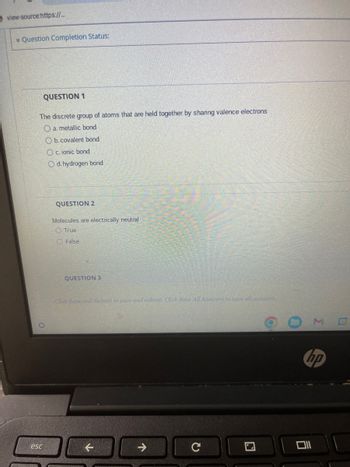
QUESTION 1
The discrete group of atoms that are held together by sharing valence electrons
O a. metallic bond
Ob. covalent bond
O c. ionic bond
Od. hydrogen bond
O
esc
QUESTION 2
Molecules are electrically neutral
True
O False
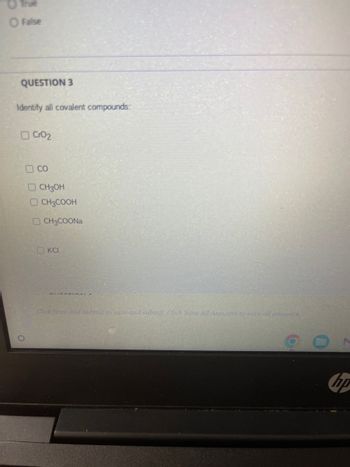
QUESTION 3
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
←
с
6
➤
hp
G
Transcribed Image Text:O False
QUESTION 3
Identify all covalent compounds:
CrO2
со
CH3OH
CH3COOH
CH3COONa
KCI
Click Save and Submit to save and submit. Click Save All Answers to save all answers,
Expert Solution
arrow_forward
Step 1
Valence electrons are the outermost electrons in an atom, and they are involved in chemical bonding. These electrons occupy the highest principal energy level (also known as the valence shell) of the atom and determine its chemical properties.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- < Tap here or pull up for additional resources - H Aktiv Learning Question 3 of 14 A skeletal structure for perchlorate (CIO) is shown below. Starting from this structure, complete the Lewis structure that follows the octet rule on all atoms. A O 60-00 CI a Click to edit mecule 111 O Tue Jun 20 2:45 PM Submit RFarrow_forwardProfiles Tab Window Help Worl x Para X cs....pdf ignment/takeCovalentActivity.do?locator=assignment-take Complete the following table: G Gran X Cr2+ Pb2+ Submit Answer NH4+ Cation Formula Anion Formula Compound Formula 14 World view.pdf My X Retry Entire Group átv SO4²- OWL X CI F How X C|Che X N. SOL X 9 more group attempts remaining Previous Next Show All ZU + X Warrow_forwardO ATOMS, IONS AND MOLECULES Understanding the difference between a molecular and empiric... What are the molecular and empirical chemical formulas of a compound made up of these molecules? H H H H HÖ-C-C=C—C—Ö-H esc H molecular formula: 0 0 empirical formula: Explanation 1 H FI Check # 80 F3 The lines stand for chemical bonds between the atoms. You can ignore the dots -- they represent "lone pairs" and you'll learn about them later. X $ F4 5 % F5 MacBook Air A & F7 ©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility DI 10/3 FB F10 C + F12arrow_forward
- Using Professor Clements' rules for bond polarity (not an electronegativity table), in a bond between F and O (F-O), what can be said about the polarity of the bond and which direction the dipole arrow points? a. The bond is polar and the dipole points towards the F atom b. The bond is non-polar and has no dipole. c. The bond is polar and the dipole points towards the O atom. d. The bond is non-polar and the dipole points towards the O atom. e. The bond is non-polar and the dipole points towards the F atom.arrow_forwardquestion 7arrow_forwardFormat Tools Table Window Help 5 Design Layout • Α' Α ab X₂ x² A 16 12. 13. V NOV References Mailings Po i 11. The bond in NaF is Aav DA a. K a. Non-polar covalent b. Polar covalent c. Ionic Name the following compounds: a. CC14 b. Fe2O3 c. SF2 d. H₂O English (United States) TEST2AFALL2022 Review View ✓ S Accessibility: Good to go Tell me Please check to see if the following are balanced. If they are not, please balance them. + Br2 KBr tv ♫♬ ↓ ¶ AaBbCcDdE. AaBbCcDdE No Spacing Normal FO ů Share Aa BbCc Headingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY