W, X, Y and Z are four different metals commonly found in school laboratory. The table below lists the results of two experiments carried out using the metals or their oxides. Experiment Addition of metal no observable to iron(II) nitrate change solution Action of heat on oxide of metal W X Y formation of a colourless gas and change a clear solution no observable |change no observable formation of silvery grey coating on Z no observable change no observable change formation of Igreyish residue (a) Which is the least reactive metal? Explain briefly. (b) Explain why a colourless gas is formed when metal X is added to iron(II) nitrate solution. Write a chemical equation, including state symbols, for the reaction involved. (c) Name the type of reaction that takes place when metal Z is added to iron(II) nitrate solution. (d) What is the most suitable method for extracting metal W from its oxide ore? Write an equation for the reaction involved, assuming that the formula of the oxide of W is WO. (e) Arrange the four given metals, as well as iron, in descending order of reactivity. 2.
W, X, Y and Z are four different metals commonly found in school laboratory. The table below lists the results of two experiments carried out using the metals or their oxides. Experiment Addition of metal no observable to iron(II) nitrate change solution Action of heat on oxide of metal W X Y formation of a colourless gas and change a clear solution no observable |change no observable formation of silvery grey coating on Z no observable change no observable change formation of Igreyish residue (a) Which is the least reactive metal? Explain briefly. (b) Explain why a colourless gas is formed when metal X is added to iron(II) nitrate solution. Write a chemical equation, including state symbols, for the reaction involved. (c) Name the type of reaction that takes place when metal Z is added to iron(II) nitrate solution. (d) What is the most suitable method for extracting metal W from its oxide ore? Write an equation for the reaction involved, assuming that the formula of the oxide of W is WO. (e) Arrange the four given metals, as well as iron, in descending order of reactivity. 2.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter22: Inorganic Materials
Section: Chapter Questions
Problem 40AP
Related questions
Question
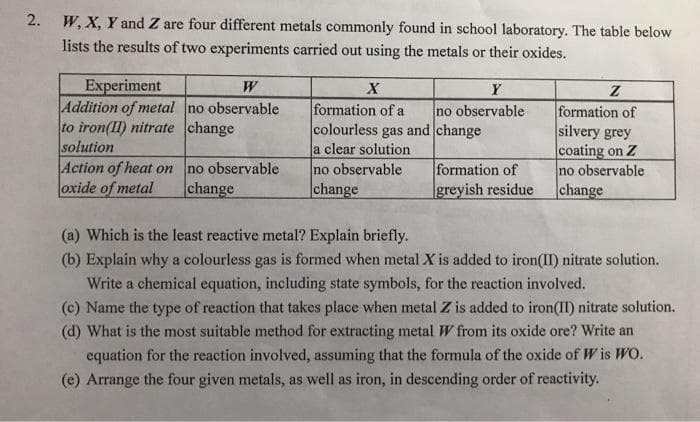
Transcribed Image Text:W, X, Y and Z are four different metals commonly found in school laboratory. The table below
lists the results of two experiments carried out using the metals or their oxides.
2.
Experiment
Addition of metal no observable
to iron(II) nitrate change
solution
Action of heat on
oxide of metal
W
X
Y
formation of a
colourless gas and change
a clear solution
no observable
change
no observable
formation of
silvery grey
coating on Z
no observable
change
no observable
change
formation of
Igreyish residue
(a) Which is the least reactive metal? Explain briefly.
(b) Explain why a colourless gas is formed when metal X is added to iron(II) nitrate solution.
Write a chemical equation, including state symbols, for the reaction involved.
(c) Name the type of reaction that takes place when metal Z is added to iron(II) nitrate solution.
(d) What is the most suitable method for extracting metal W from its oxide ore? Write an
equation for the reaction involved, assuming that the formula of the oxide of W is WO.
(e) Arrange the four given metals, as well as iron, in descending order of reactivity.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning