Weicome students Mancopa X O Quiz 11 (CHEM101) Chem101 i app.101edu.co Question 1 of 29 Convert 2.70 atm to torr STARTING AMOUNT ADO FACTOR ANSWER RESET 1000 101.325 20.3 14.70 1.01325 0.00355 1.01325 x 10 10 2050 760 2.70 0.001 0.98692 kPa Pa mm Hg torr bar atm psi MacBook Air 80 DD esc F2 F3 23 & 4 7 9. . CO CO
Weicome students Mancopa X O Quiz 11 (CHEM101) Chem101 i app.101edu.co Question 1 of 29 Convert 2.70 atm to torr STARTING AMOUNT ADO FACTOR ANSWER RESET 1000 101.325 20.3 14.70 1.01325 0.00355 1.01325 x 10 10 2050 760 2.70 0.001 0.98692 kPa Pa mm Hg torr bar atm psi MacBook Air 80 DD esc F2 F3 23 & 4 7 9. . CO CO
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter4: Introduction To Gases
Section: Chapter Questions
Problem 67E: The compression ratio in an automobile engine is the ratio of the gas pressure at the end of the...
Related questions
Question
What are the answers to these questions
Transcribed Image Text:2 Welcome Students | Maricopa x
O Quiz 11 (CHEM101)
o Chem101
i app.101edu.co
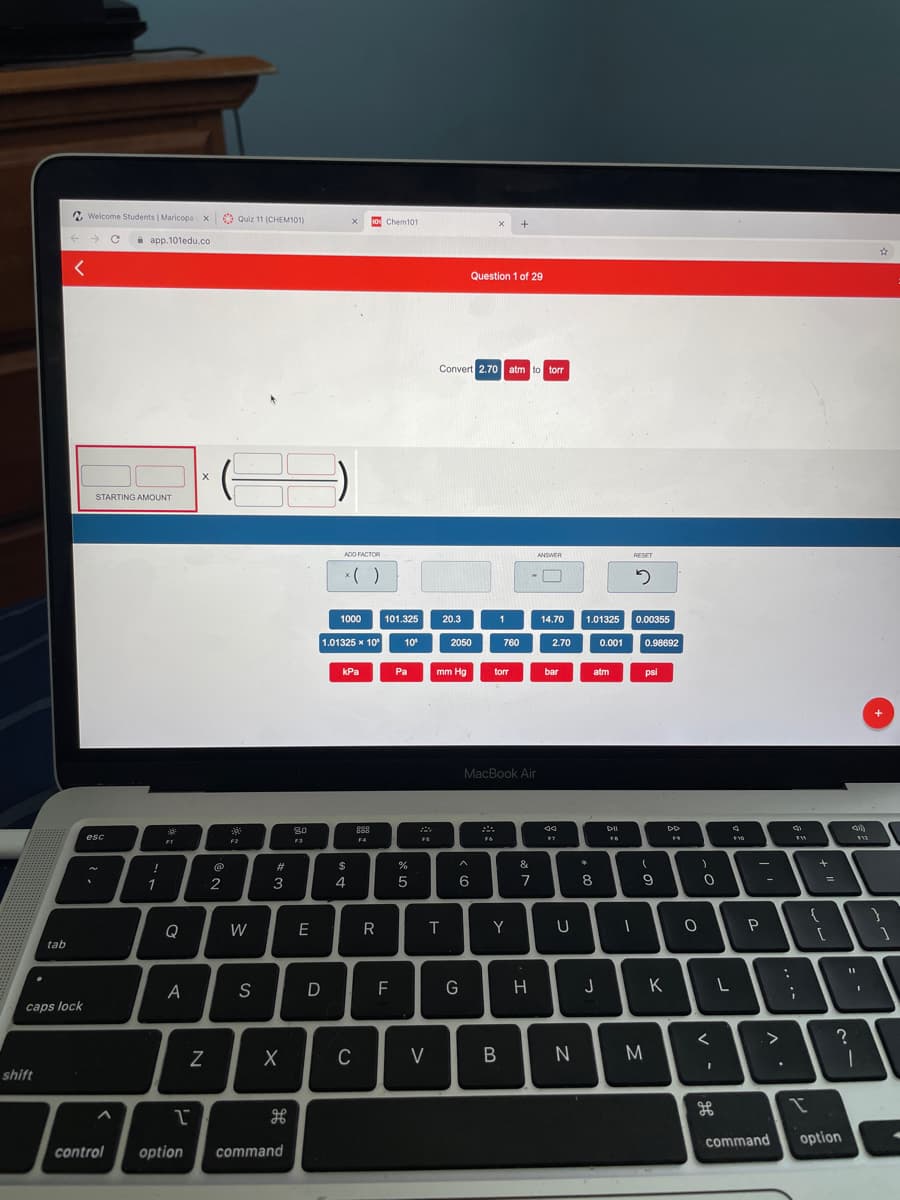
Question 1 of 29
Convert 2.70 atm to torr
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
1000
101.325
20.3
1
14.70
1.01325
0.00355
1.01325 x 10
10
2050
760
2.70
0.001
0.98692
kPa
Pa
mm Hg
torr
bar
atm
psi
MacBook Air
80
DI
I
esc
F4
FS
FI
F2
F3
@
23
$
&
*
1
2
3
4
7
8
Q
W
E
R
T
Y
P
tab
S
H
K
caps lock
C
V
M
shift
command
option
control
option
сommand
N
Transcribed Image Text:Welcome Students | Maricopa X
6 Quiz 11 (CHEM101)
soi Chem101
i app.101edu.co
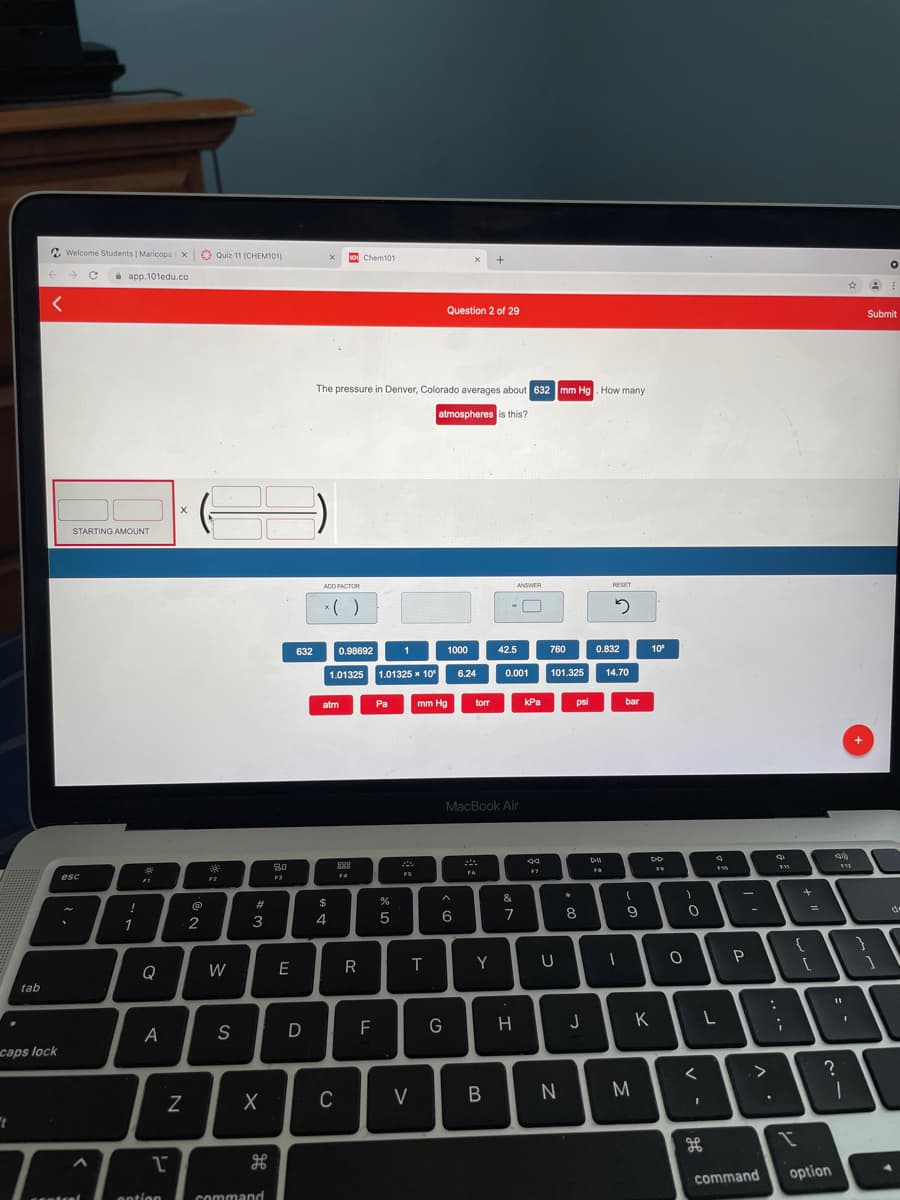
Question 2 of 29
Submit
The pressure in Denver, Colorado averages about 632 mm Hg. How many
atmospheres is this?
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
* ( )
632
0.98692
1
1000
42.5
760
0.832
10
1.01325 1.01325 x 10
0.001
101.325
6.24
14.70
atm
Pa
mm Hg
torr
kPa
psi
bar
+
MacBook Air
**
888
F7
FS
esc
F4
F2
&
#3
$
8
9
%3D
de
-|
2
3
4
6
7
1
Q
W
E
R
T
Y
tab
D
F
H
J
K
A
S
caps lock
C
V
M
command
option
antion
command
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning