What is the concentration of carnosine in your cell lysate?
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
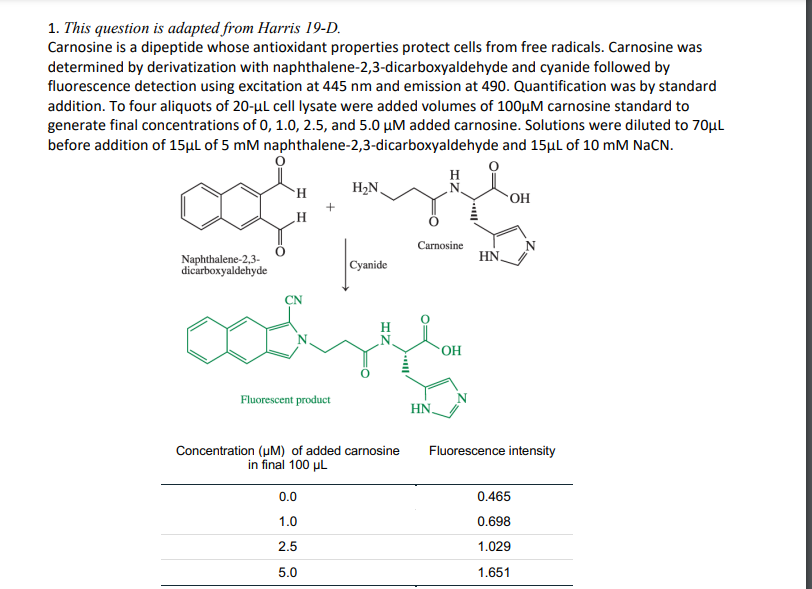
Transcribed Image Text:1. This question is adapted from Harris 19-D.
Carnosine is a dipeptide whose antioxidant properties protect cells from free radicals. Carnosine was
determined by derivatization with naphthalene-2,3-dicarboxyaldehyde and cyanide followed by
fluorescence detection using excitation at 445 nm and emission at 490. Quantification was by standard
addition. To four aliquots of 20-ul cell lysate were added volumes of 100µM carnosine standard to
generate final concentrations of 0, 1.0, 2.5, and 5.0 µM added carnosine. Solutions were diluted to 70µl
before addition of 15µl of 5 mM naphthalene-2,3-dicarboxyaldehyde and 15µl of 10 mM NaCN.
H
H2N.
OH
Carnosine
HN.
Naphthalene-2,3-
dicarboxyaldehyde
Cyanide
OH
Fluorescent product
HN.
Fluorescence intensity
Concentration (uM) of added carnosine
in final 100 µL
0.0
0.465
1.0
0.698
2.5
1.029
5.0
1.651

Transcribed Image Text:c) What is the concentration of carnosine in your cell lysate? If you use excel at any point,
please include a copy of your workings.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 13 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON