What volume of 0.1M NaOH (reagent) needs to be added to increase your 7.5 pH (buffer) by 0.5 pH units. The goal is to further purify MOPs (see image). Protonated form = 6.6 x 10.3^-3 mol/L Deprotonated form = 1.32 x 10^-2 mol/L Show calculations.
What volume of 0.1M NaOH (reagent) needs to be added to increase your 7.5 pH (buffer) by 0.5 pH units. The goal is to further purify MOPs (see image). Protonated form = 6.6 x 10.3^-3 mol/L Deprotonated form = 1.32 x 10^-2 mol/L Show calculations.
Case Studies In Health Information Management
3rd Edition
ISBN:9781337676908
Author:SCHNERING
Publisher:SCHNERING
Chapter2: Information Protection: Access, Archival, Privacy, And Security
Section: Chapter Questions
Problem 2.8.11C
Related questions
Question
What volume of 0.1M NaOH (reagent) needs to be added to increase your 7.5 pH (buffer) by 0.5 pH units. The goal is to further purify MOPs (see image).
Protonated form = 6.6 x 10.3^-3 mol/L
Deprotonated form = 1.32 x 10^-2 mol/L
Show calculations.
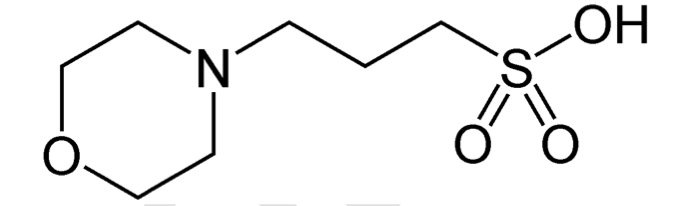
Transcribed Image Text:HO
N.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Case Studies In Health Information Management
Biology
ISBN:
9781337676908
Author:
SCHNERING
Publisher:
Cengage

Medical Terminology for Health Professions, Spira…
Health & Nutrition
ISBN:
9781305634350
Author:
Ann Ehrlich, Carol L. Schroeder, Laura Ehrlich, Katrina A. Schroeder
Publisher:
Cengage Learning


Case Studies In Health Information Management
Biology
ISBN:
9781337676908
Author:
SCHNERING
Publisher:
Cengage

Medical Terminology for Health Professions, Spira…
Health & Nutrition
ISBN:
9781305634350
Author:
Ann Ehrlich, Carol L. Schroeder, Laura Ehrlich, Katrina A. Schroeder
Publisher:
Cengage Learning

