Which one of the equations below represents what happens when HC2H3O2 is dissolved in water? O HC2H3O2+H20 C2H3O2 (aq) + OH (aq) O HC,H;02 + H,o H;O*(aq) + C,H;O2 (aq) O HC2H3O2+ H20 H3O(aq) + C2H3O2*(aq) O HC2H;O2+H20 2H (aq) +OH (aq) + C2H3O2 (aq) O HC,H;O2+ H20 H3O*(aq) + C2H3O2*(aq)
Which one of the equations below represents what happens when HC2H3O2 is dissolved in water? O HC2H3O2+H20 C2H3O2 (aq) + OH (aq) O HC,H;02 + H,o H;O*(aq) + C,H;O2 (aq) O HC2H3O2+ H20 H3O(aq) + C2H3O2*(aq) O HC2H;O2+H20 2H (aq) +OH (aq) + C2H3O2 (aq) O HC,H;O2+ H20 H3O*(aq) + C2H3O2*(aq)
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 100E: Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a...
Related questions
Question
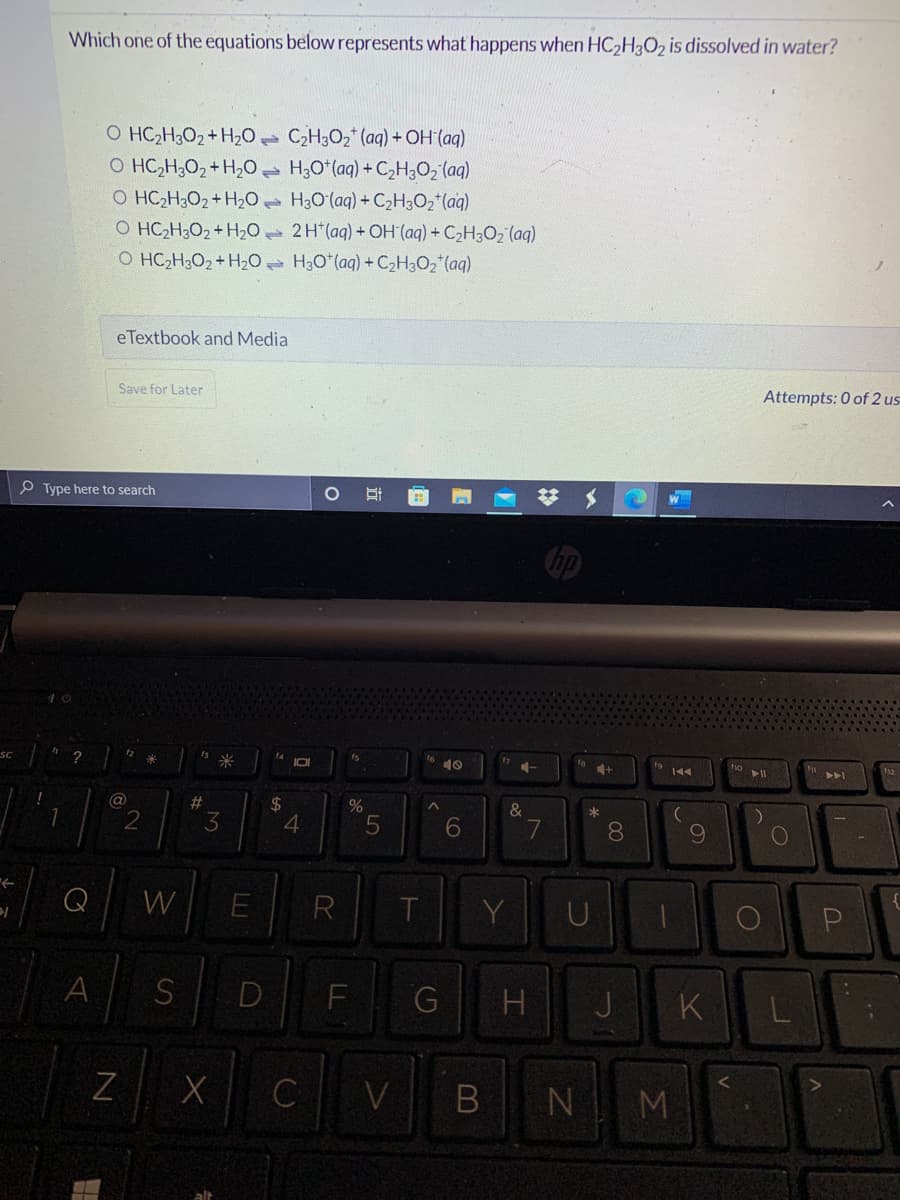
Transcribed Image Text:Which one of the equations below represents what happens when HC2H3O2 is dissolved in water?
O HC2H3O2+H20 C2H3O2 (aq) + OH (aq)
O HC,H;0, + H,0 H;O*(aq) + C,H;02 (aq)
O HC2H3O2+ H20 H30(aq) + C2H3O2*(aq)
O HC2H;O2+ H20 2H*(aq) + OH (aq) + C2H3O2 (aq)
O HC2H3O2+H20 H3O*(aq) + C2H3O2 (aq)
eTextbook and Media
Save for Later
Attempts: 0 of 2 us
P Type here to search
SC
米
10
to
144
11
トト」
%23
%24
&
3
4
8.
Q
W
R
T
Y
A
D
G
H.
J
K
L
V
B N M
LL
Expert Solution
Step 1
Acids give H+ ions and bases gain H+ ions.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning