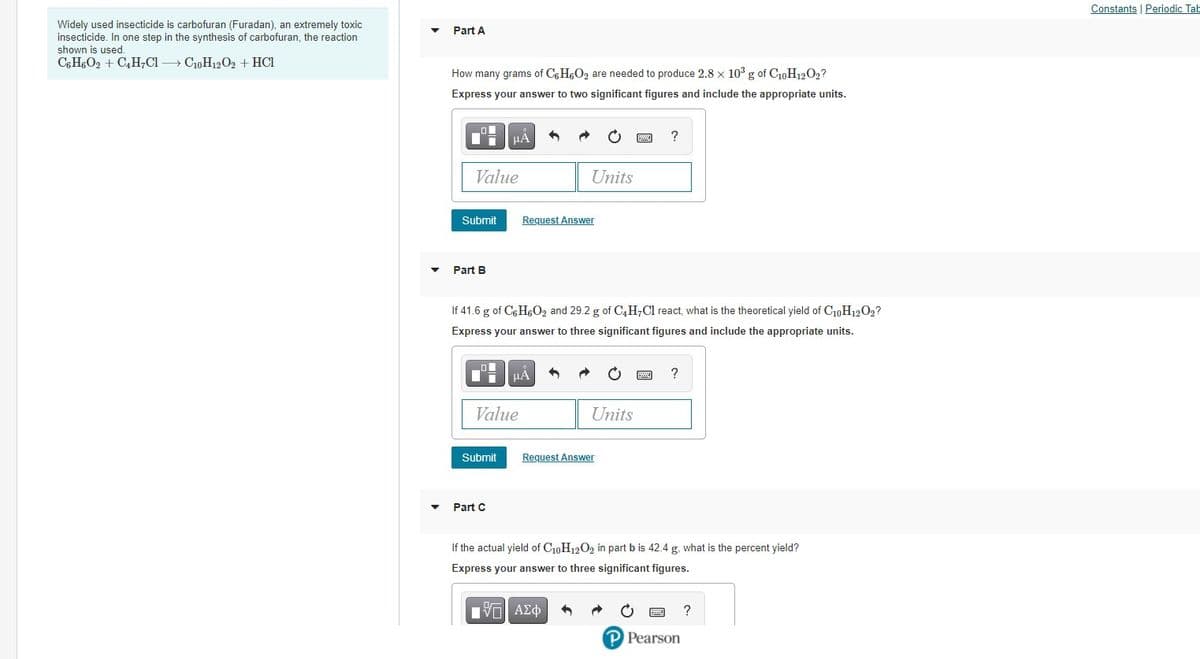
Widely used insecticide is carbofuran (Furadan), an extremely toxic insecticide. In one step in the synthesis of carbofuran, the reaction shown is used Part A CHO2 + C,H;CI CoH12O2 + HCı How many grams of CHeO2 are needed to produce 2.8 x 10° g of CoH20,? Express your answer to two significant figures and include the appropriate units. HẢ Value Units Submit Request Answer Part B If 41.6 g of CH,Oz and 29.2 g of C,H,Cl react what is the theoretical yield of C0H1202? Express your answer to three significant figures and include the appropriate units. HÀ ? Value Units Submit Request Answer Part C if the actual yield of C10H12O2 in part b is 42.4 g. what is the percent yield? Express your answer to three significant figures.
Widely used insecticide is carbofuran (Furadan), an extremely toxic insecticide. In one step in the synthesis of carbofuran, the reaction shown is used Part A CHO2 + C,H;CI CoH12O2 + HCı How many grams of CHeO2 are needed to produce 2.8 x 10° g of CoH20,? Express your answer to two significant figures and include the appropriate units. HẢ Value Units Submit Request Answer Part B If 41.6 g of CH,Oz and 29.2 g of C,H,Cl react what is the theoretical yield of C0H1202? Express your answer to three significant figures and include the appropriate units. HÀ ? Value Units Submit Request Answer Part C if the actual yield of C10H12O2 in part b is 42.4 g. what is the percent yield? Express your answer to three significant figures.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.77PAE: The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce...
Related questions
Question
Transcribed Image Text:Constants | Periodic Tab
Widely used insecticide is carbofuran (Furadan), an extremely toxic
insecticide. In one step in the synthesis of carbofuran, the reaction
shown is used.
C6 H6O2 + C4H7C1 » C10H1202 + HCl
Part A
How many grams of C6 H6O2 are needed to produce 2.8 x 103 g of C10H12O2?
Express your answer to two significant figures and include the appropriate units.
HA
Value
Units
Submit
Request Answer
Part B
If 41.6 g of C6 H6O2 and 29.2 g of C,H;Cl react, what is the theoretical yield of C10H1202?
Express your answer to three significant figures and include the appropriate units.
HA
?
Value
Units
Submit
Request Answer
Part C
If the actual yield of C10H1202 in part b is 42.4 g, what is the percent yield?
Express your answer to three significant figures.
?
P Pearson
圓
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning