You are trying to determine the caffeine content of brand X coffee using HPLC-UV/VIS analysis. You used a C18 column and a water : methanol mobile phase of 60:40 ratio. The detector was set to measure at 275 nm. Fill the blanks in the following table using the correct number of significant figures. Use whole numbers only. Area (uV'sec) Concentration (ppm) Standard Deviation Trial 1 Trial 2 Trial 3 Average (use 3 S.F.) 2.00 69400 70400 69900 4.00 110525 109415 110390 6.00 145467 143405 145759 8.00 197899 196474 197196 10.00 243151 242193 241691
You are trying to determine the caffeine content of brand X coffee using HPLC-UV/VIS analysis. You used a C18 column and a water : methanol mobile phase of 60:40 ratio. The detector was set to measure at 275 nm. Fill the blanks in the following table using the correct number of significant figures. Use whole numbers only. Area (uV'sec) Concentration (ppm) Standard Deviation Trial 1 Trial 2 Trial 3 Average (use 3 S.F.) 2.00 69400 70400 69900 4.00 110525 109415 110390 6.00 145467 143405 145759 8.00 197899 196474 197196 10.00 243151 242193 241691
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter1: Introduction
Section: Chapter Questions
Problem 1.10QAP
Related questions
Question
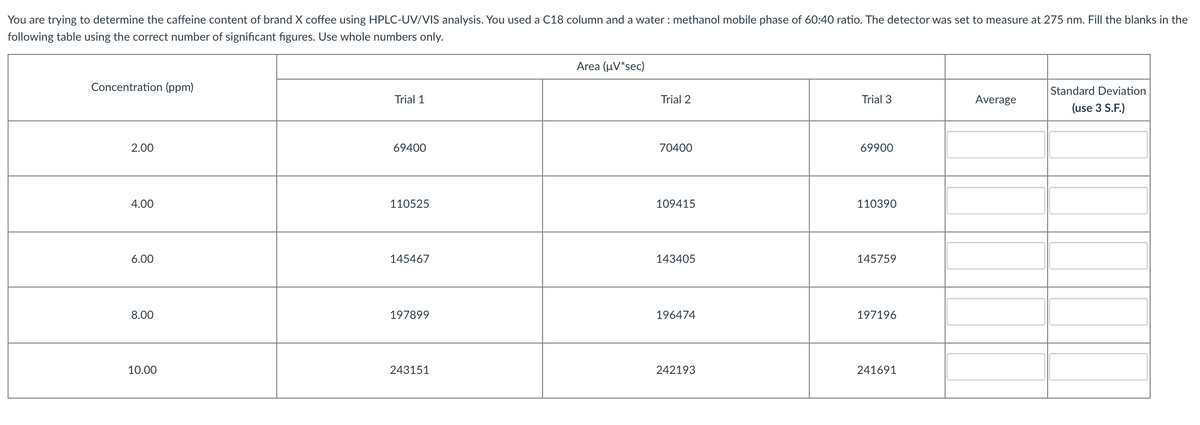
Transcribed Image Text:You are trying to determine the caffeine content of brand X coffee using HPLC-UV/VIS analysis. You used a C18 column and a water : methanol mobile phase of 60:40 ratio. The detector was set to measure at 275 nm. Fill the blanks in the
following table using the correct number of significant figures. Use whole numbers only.
Area (μν"sec)
Concentration (ppm)
Standard Deviation
Trial 1
Trial 2
Trial 3
Average
(use 3 S.F.)
2.00
69400
70400
69900
4.00
110525
109415
110390
6.00
145467
143405
145759
8.00
197899
196474
197196
10.00
243151
242193
241691
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning