AA (mg/g) UV (ug/ml) Sample (ug/ml) Unknown Fe solution 1 Unknown Fe solution _2 0.322 8.136 0.318 7.967 Unknown Fe solution_3 0.32 8.339 Mean Standard deviation 95% Confidence limit (use t=4.3)
AA (mg/g) UV (ug/ml) Sample (ug/ml) Unknown Fe solution 1 Unknown Fe solution _2 0.322 8.136 0.318 7.967 Unknown Fe solution_3 0.32 8.339 Mean Standard deviation 95% Confidence limit (use t=4.3)
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
100%
calculate mean,SD and 95%confidence limit. Use t=4.3.Compare the two means using the student's t test and precision of the two methods using F test at 95% confidence level . For two sets of three replicates use t=2.78 and fcrit=19
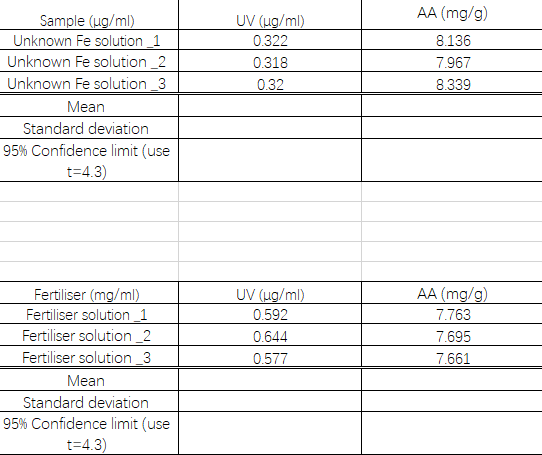
Transcribed Image Text:AA (mg/g)
UV (ug/ml)
Sample (ug/ml)
Unknown Fe solution 1
Unknown Fe solution _2
0.322
8.136
0.318
7.967
Unknown Fe solution_3
0.32
8.339
Mean
Standard deviation
95% Confidence limit (use
t=4.3)
AA (mg/g)
Fertiliser (mg/ml)
Fertiliser solution 1
Fertiliser solution_2
Fertiliser solution_3
UV (ug/ml)
0.592
7.763
0.644
7.695
0.577
7.661
Mean
Standard deviation
95% Confidence limit (use
t=4.3)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



