
Concept explainers
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
(a)
Interpretation:
The molecular art has to be classified as a pure element, a pure compound or a mixture.
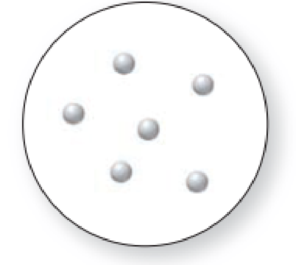
The given molecular art is,
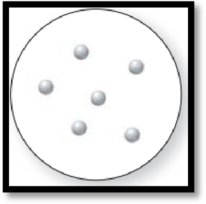
Figure 1
Concept Introduction:
Matter can be classified into two type’s namely pure substance and mixture.
Pure substance: A single component that has a constant composition, irrespective of the sample size and the sample origin is called as pure substance. A pure substance could not be broken down to other pure substances by any physical change.
Example: Water, sugar etc.
Element: A pure substance, which cannot be broken down into smaller substances by a chemical reaction is called as an element.
Example: Hydrogen gas, Magnesium ribbon and copper wire etc.
Mixture: A mixture consists of more than one substance and the composition of a mixture is dependent on the sample. The separation of mixture into its components can be done by physical changes.
Compound: A pure substance that is formed by combination of two or more elements by chemical process is called as a compound. Example: Sodium chloride is a compound because it is formed from elements sodium and chlorine.
Answer to Problem 1.31UKC
The given molecular art is a pure element.
Explanation of Solution
The given molecular art is,
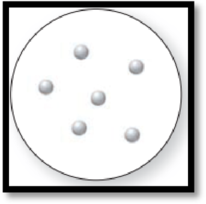
Figure 1
The molecular art shown above is a pure element because it comprises of only single entity.
(b)
Interpretation:
The molecular art has to be classified as a pure element, a pure compound or a mixture.
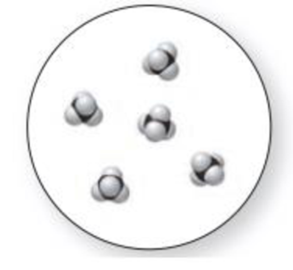
The given molecular art is,
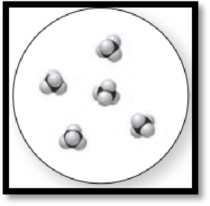
Figure 2
Concept Introduction:
Refer to part (a).
Answer to Problem 1.31UKC
The given molecular art is a pure compound.
Explanation of Solution
The given molecular art is,
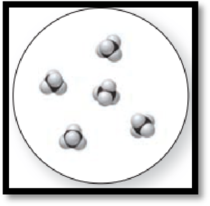
Figure 2
The given molecular art is a pure compound because it comprises of combination of two elements (one type of molecules).
(c)
Interpretation:
The molecular art has to be classified as a pure element, a pure compound or a mixture.
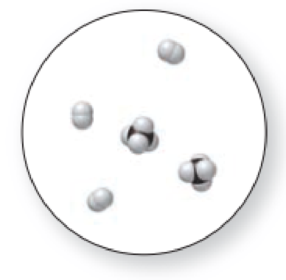
The given molecular art is,
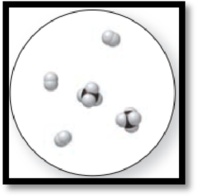
Figure 3
Concept Introduction:
Refer to part (a).
Answer to Problem 1.31UKC
The given molecular is a mixture.
Explanation of Solution
The given molecular art is,
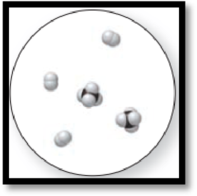
Figure 3
The molecular art shown above is a mixture because it comprises of more than one component (different types of molecules).
(d)
Interpretation:
The molecular art has to be classified as a pure element, a pure compound or a mixture.
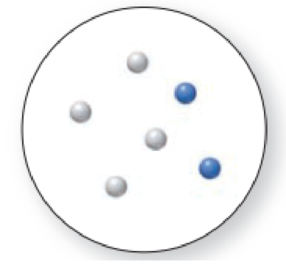
The given molecular art is,
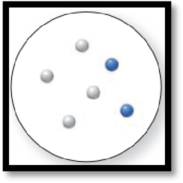
Figure 4
Concept Introduction:
Refer to part (a).
Answer to Problem 1.31UKC
The given molecular art is a mixture.
Explanation of Solution
The given molecular art is,
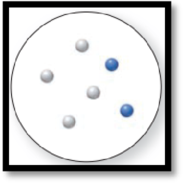
Figure 4
The molecular art shown above is a mixture because it consists of more than a single component (different types of atoms).
Want to see more full solutions like this?
Chapter 1 Solutions
PRIN.OF GENERAL,ORGANIC+BIOLOG.CHEM.
Additional Science Textbook Solutions
Chemistry
Living by Chemistry
Organic Chemistry As a Second Language: Second Semester Topics
CHEMISTRY-TEXT
- If iron filings are placed with excess powdered sulfur in a beaker, the iron filings are still attracted by a magnet and could be separated from the sulfur with the magnet. Would this combination of iron and sulfur represent amixtureor apure substance?arrow_forwardHow does an element differ from a compound? How are they similar?arrow_forwardClassifying Matter Determine whether each of the following is a pure element, a compound, or a mixture. If it is a mixture, classify it as homogeneous or heterogeneous: a. pure salt b. helium gas c. chicken noodle soup d. coffeearrow_forward
- Which of the following are elements, and which are compounds? a NaOH; b BaCl2; c He; d Ag; e Fe2O3.arrow_forwardClassify each of the following as an element, a compound, or a mixture: (a) copper (b) water (c) nitrogen (d) sulfur (e) air (f) sucrose (g) a substance composed of molecules each of which contains two iodine atoms (h) gasolinearrow_forwardWhich of the following particulate illustrations represent pure substances and which represent mixtures?arrow_forward
- Diamonds and graphite are two forms of carbon. Carbon is an element. Chunks of graphite are sprinkled among the diamonds on a jewelers display tray. Is the material on the tray a pure substance or a mixture? Is the display homogenous or heterogeneous? Justify both answers.arrow_forwardA cup of coffee is an example of: a. a liquid pure substance b. a gaseous mixture c. a solid pure substance d. a liquid mixture e. a solid mixturearrow_forwardClassify each of the following pure substances as either an element or a compound: a C; b C2H5OH; c Cl2.arrow_forward
- 1f a piece of hard, white blackboard chalk is heated strongly in a flame, the mass of the piece of chalk will decrease, and eventually the chalk will crumble into a white dust. Does this change suggest that the chalk is composed of an element or a compound?arrow_forwardWhich of the following contains an element, a compound, and a mixture? copper, silicon dioxide(SiO2) , copper(II) sulfate(CuSO4) hydrogen, carbon dioxide(CO2) , water(H2O) chili, pizza, steak sodium, sodium chloride (NaCl). salt water nitrogen, argon, airarrow_forward1.74 What are the two properties of ITO that make it serve its function in touch screen applications?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





