
Concept explainers
(a)
Interpretation:
The volume of a bacterial cell in cubic millimeters
Concept introduction:
Volume is a physical quantity and its SI unit is cubic meter
The conversion of one unit into another can be done using a proper conversion factor. Conversion factors are the ratios that relate the two different units of a quantity. It is also known as dimensional analysis or factor label method.
In the unit conversion problems, the given information is multiplied by the conversion factors to obtain the desired information. The unit conversion can be done as follows:
(a)
Answer to Problem 1.36P
The volume of a bacterial cell in cubic millimeters
Explanation of Solution
The volume of a bacterial cell in cubic micrometer
The road map to calculate the volume of a bacterial cell in cubic millimeters
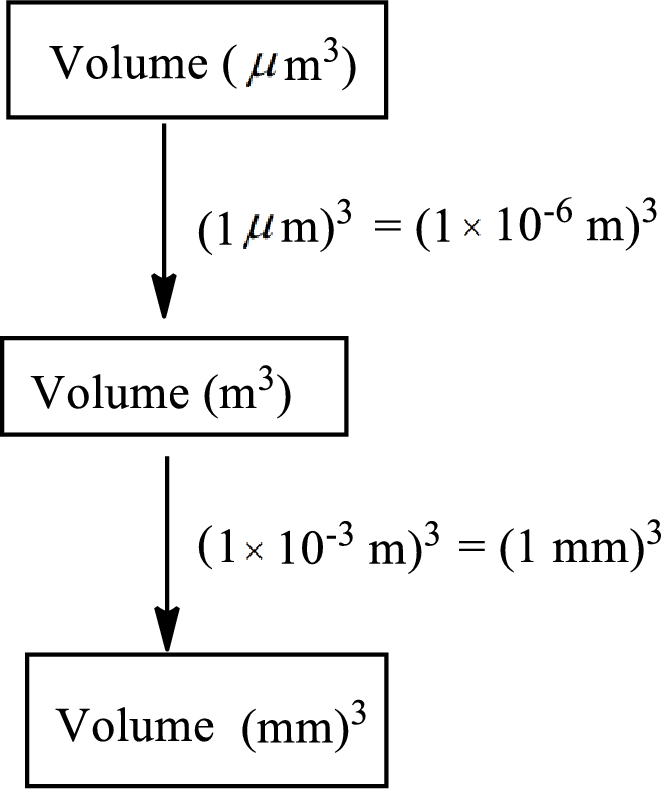
The conversion factor to convert volume from cubic micrometer
The conversion factor to convert volume from cubic meter
Convert the volume from cubic micrometer
The volume of a bacterial cell in cubic millimeters
(b)
Interpretation:
The volume of
Concept introduction:
Volume is a physical quantity and its SI unit is cubic meter
The conversion of one unit into another can be done using a proper conversion factor. Conversion factors are the ratios that relate the two different units of a quantity. It is also known as dimensional analysis or factor label method.
In the unit conversion problems, the given information is multiplied by the conversion factors to obtain the desired information. The unit conversion can be done as follows:
(b)
Answer to Problem 1.36P
The volume of
Explanation of Solution
The volume of a bacterial cell in cubic micrometer
The road map to calculate the volume of
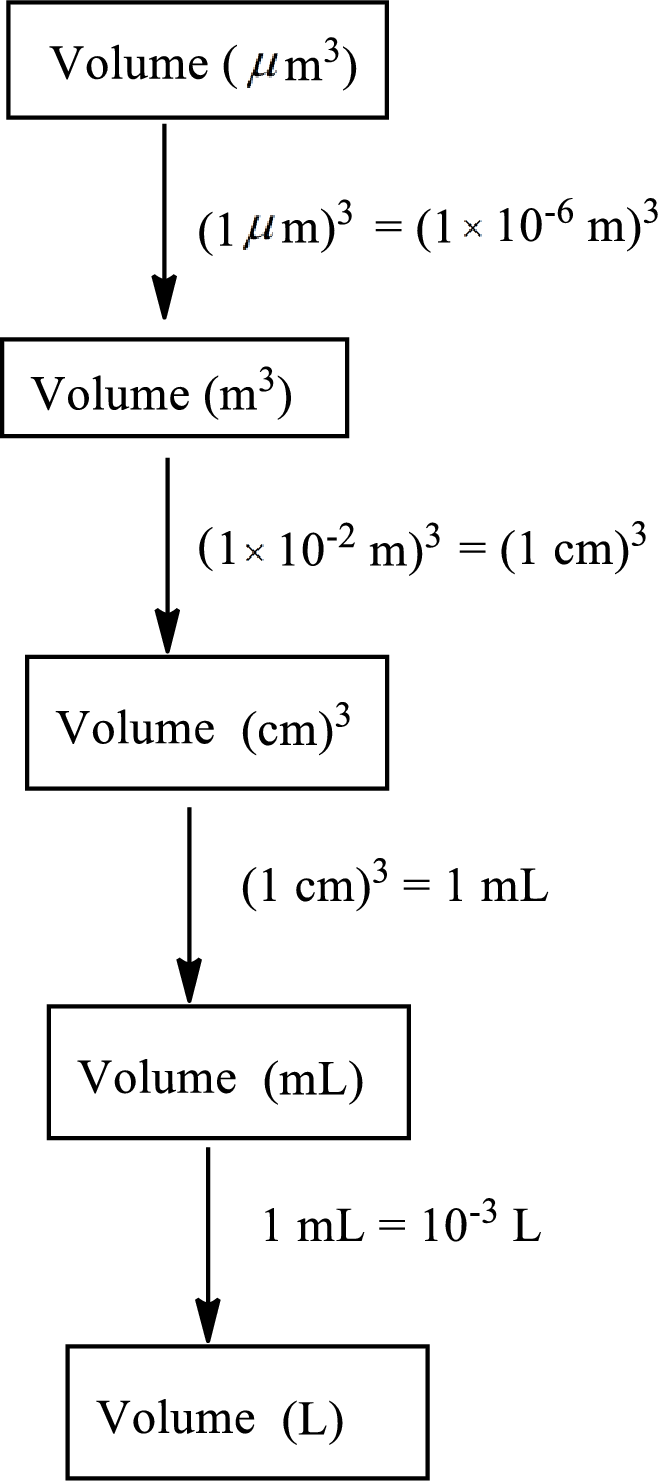
The conversion factor to convert volume from cubic micrometer
The conversion factor to convert volume from cubic meter
The conversion factor to convert volume from cubic centimeters
The conversion factor to convert volume from milliliters
Convert the volume from cubic micrometer
Calculate the volume of
The volume of
Want to see more full solutions like this?
Chapter 1 Solutions
GEN CMB CHEM; CNCT+;ALEKS 360
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





