
Concept explainers
(a)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
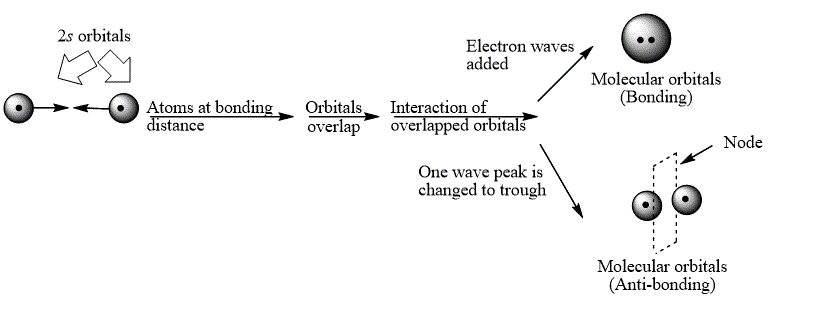
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two

Figure 1
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(b)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
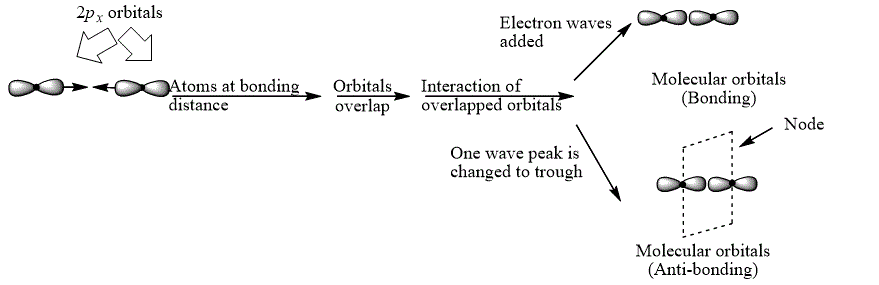
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two
 Figure 2
Figure 2
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(c)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
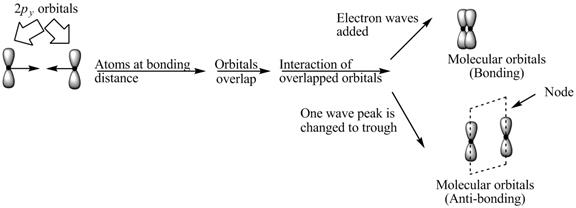
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two
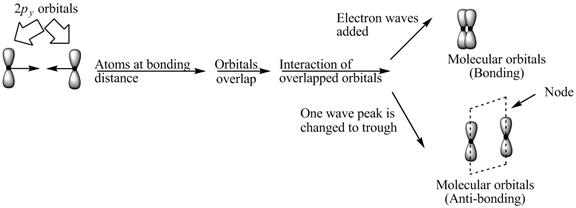
Figure 3
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(d)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
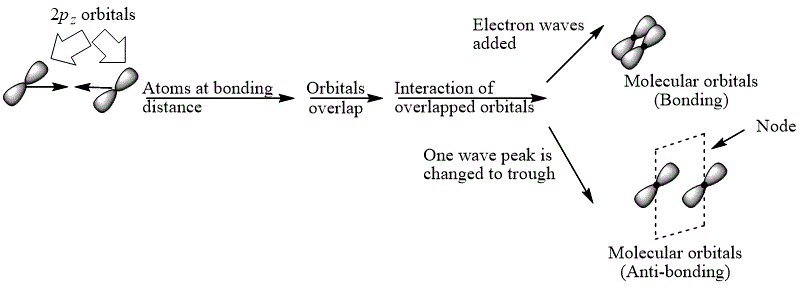
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two

Figure 4
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(e)
Interpretation:
The orbital interaction energy diagram showing the energy levels of the atomic orbitals along with the energies of the given MOs is to be drawn.
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The orbital interaction energy diagram showing the energy levels of the atomic orbitals along with the energies of the given MOs is represented below.
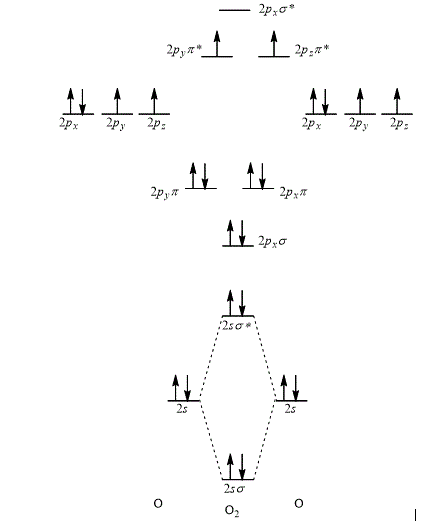
Explanation of Solution
According to the rules for filling the electrons in the molecular orbital, first of all the lower energy molecular orbital is filled followed by the filling of increasing energy order of the molecular orbital.
Thus, the orbital interaction energy diagram showing the energy levels of the atomic orbitals along with the energies of the given MOs of oxygen atom is shown as,

Figure 5
The energy of sigma,
The orbital interaction energy diagram showing the energy level of the atomic orbitals along with the energies of the given MOs is shown Figure 5.
(f)
Interpretation:
The reason corresponding to the fact that liquid
Concept introduction:
The atoms which contain no unpaired electron in their orbital and have total spin equals to zero are known as diamagnetic atoms. The atoms which contain one or more unpaired electron in their orbital are known as paramagnetic atoms.
Answer to Problem 1.47AP
The liquid
Explanation of Solution
In oxygen molecule, two unpaired electrons are present in the anti-bonding molecular orbital. Thus, oxygen molecule is paramagnetic in nature due to which it gets attracted towards the magnetic field.
In comparison to liquid oxygen, the movement of the oxygen gas molecules is faster. Therefore, liquid oxygen molecules are not attracted by the magnetic field.
Hence, liquid
The reason corresponding to the fact that liquid
(g)
Interpretation:
The Lewis structure that best describes the covalent bond(s) in
Concept introduction:
The Lewis structure shows the connectivity between atoms by identifying the lone pairs of electrons in a compound. Lewis structures are also called Lewis dot structures. The valence electrons around an atom are shown by dots. Bonds between atoms are shown by lines and the lone pair of electrons is shown by a pair of dots.
Answer to Problem 1.47AP
The Lewis structure that best describes the covalent bond(s) in
![]()
Explanation of Solution
The given Lewis structures of
The reason corresponding to the fact that liquid
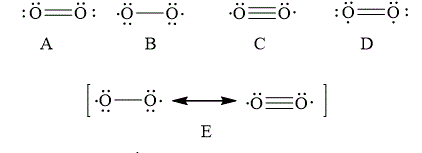
Figure 6
The total number of bonding electrons in
The total number of anti-bonding electrons in
The bond order of
Substitute the number of bonding molecular orbital and anti-bonding molecular orbital electrons in the above expression.
Thus, the bond order of oxygen molecule is
Therefore, the Lewis structures of
In option D, the Lewis structures of
In option A, the Lewis structures of
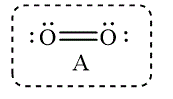
Figure 7
The Lewis structure that best describes the covalent bond(s) in
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Solid sulfur normally consists of crystals of S8 molecules, but when heated strongly, the solid vaporizes to give S2 molecules (among other molecular species). Describe the bonding in S2 in molecular orbital terms, assuming the orbitals are analogous to those of the preceding period. What would you expect to happen to the sulfur sulfur bond length if two electrons were added to give the S22 ion? What would you expect to happen to the bond length if, instead, two electrons were taken away to give S22+?arrow_forwardThe structure of amphetamine, a stimulant, is shown below. (Replacing one H atom on the NH2, or amino, group with CH3 gives methamphetamine a particularly dangerous drug commonly known as speed.) (a) What are the hybrid orbitals used by the C atoms of the C6 ring. by the C atoms of the side chain, and by the N atom? (b) Give approximate values for the bond angles A, B, and C. (c) How many bonds and bonds are in the molerule? (d) Is the molecule polar or nonpolar? (e) Amphetamine reacts readily with a proton (H+) in aqueous solution. Where does this proton attach to the molecule? Explain how the electrostatic potential map predicts this site of protonation.arrow_forwardConsider the polyatomic ion IO65-. How many pairs of electrons are around the central iodine atom? What is its hybridization? Describe the geometry of the ion.arrow_forward
- What modification to the molecular orbital model was made from the experimental evidence that B2 is paramagnetic?arrow_forwardIn the molecular orbital mode l, compare and contrast bonds with bonds. What orbitals form the bonds and what orbitals form the bonds? Assume the z-axis is the internuclear axis.arrow_forwardDescribe the hybridization around the central atom and the bonding in SCl2 and OCS.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





