
Masteringchemistry With Pearson Etext -- Valuepack Access Card -- For Organic Chemistry
9th Edition
ISBN: 9780134161600
Author: Wade
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.53SP
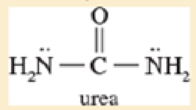
In most
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the following descriptions of molecules, draw the Lewis structure (showing all
atoms, lone pairs, formal charges) of the molecule and show all bond angles
(assuming ideal VSEPR angles).
An organic compound with the molecular formula H3CNO2. C is sp2
hybridized, N is sp3 hybridized, one O is sp² hybridized, while the second O is sp3
hybridized.
An organic compound with the molecular formula H2CNO*. Both C
and O are sp hybridized while N is sp³ hybridized.
Pyrazine, C4H4N2, is used in the synthesis of vitamins and drugs. The molecule can be
thought of replacing two -CH units in benzene with a nitrogen atom in each of the 1,4-
positions.
pyrazine
Describe the o and a bonding in pyrazine by analyzing the hybridization state,
valence electron count and o-framework.
How many delocalized electrons are present? Explain your answer.
How many bonding and anti-bonding 7-type molecular orbitals are present? Explain your
answer.
Draw the detailed MO diagram of the 7-system. Explain your answer.
Yeni Metin Belgesi - Not Defteri
Dosya Düzen Biçim Görünüm
Yardım
For acetaldehyde (CH3CHO) write the
Lewis structure. Write the hybridization
and geometry of each carbon. Draw its
3D structure and write the bond angles
(approximately).
Chapter 1 Solutions
Masteringchemistry With Pearson Etext -- Valuepack Access Card -- For Organic Chemistry
Ch. 1.2C - a. Nitrogen has relatively stable isotopes...Ch. 1.4 - Draw Lewis structures for the following compounds....Ch. 1.5 - Write Lewis structures for the following molecular...Ch. 1.5 - Circle any lone pairs (pairs of nonbonding...Ch. 1.6 - Use electronegativities to predict the direction...Ch. 1.8 - Prob. 1.6PCh. 1.9B - Draw the important resonance forms for the...Ch. 1.9B - Prob. 1.8PCh. 1.9B - Prob. 1.9PCh. 1.9B - Use resonance structures to identify the areas of...
Ch. 1.10A - Draw complete Lewis structures for the following...Ch. 1.10B - Give Lewis structures corresponding to the...Ch. 1.10B - Prob. 1.13PCh. 1.11 - Compute the empirical and molecular formulas for...Ch. 1.16 - a. Use your molecular models to make ethane, and...Ch. 1.17 - a. Predict the hybridization of the oxygen atom in...Ch. 1.17 - Predict the hybridization geometry and bond angles...Ch. 1.17 - Predict the hybridization, geometry, and bond...Ch. 1.17 - Prob. 1.19PCh. 1.17 - Allene, CH2=C=CH2, has the structure shown below...Ch. 1.17 - 1. Draw the important resonance forms for each...Ch. 1.18B - Prob. 1.22PCh. 1.18B - Two compounds with the formula CH3CH=NCH3 are...Ch. 1.19B - Prob. 1.24PCh. 1.19B - Give the relationship between the following pairs...Ch. 1 - a. Draw the resonance forms for SO2 (bonded OSO)....Ch. 1 - Name the element that corresponds to each...Ch. 1 - Prob. 1.28SPCh. 1 - For each compound, state whether its bonding is...Ch. 1 - a. Both PCl3 and PCl5 are stable compounds Draw...Ch. 1 - Draw a Lewis structure for each species a. N2H4 b....Ch. 1 - Prob. 1.32SPCh. 1 - Prob. 1.33SPCh. 1 - Draw Lewis structures for a. two compounds of...Ch. 1 - Prob. 1.35SPCh. 1 - Some of the following molecular formulas...Ch. 1 - Prob. 1.37SPCh. 1 - Give the molecular formula of each compound shown...Ch. 1 - 1. From what you remember of electronegativities,...Ch. 1 - For each of the following structures, 1. Draw a...Ch. 1 - Prob. 1.41SPCh. 1 - Prob. 1.42SPCh. 1 - Prob. 1.43SPCh. 1 - Prob. 1.44SPCh. 1 - For each pair of ions, determine which on is more...Ch. 1 - Use resonance structures to identify the areas of...Ch. 1 - Prob. 1.47SPCh. 1 - In 1934, Edward A. Doisy of Washington University...Ch. 1 - If the carbon atom in CH2Cl2 were fat. there would...Ch. 1 - Cyclopropane (C3H6, a three-membered ring) is more...Ch. 1 - Prob. 1.51SPCh. 1 - Prob. 1.52SPCh. 1 - In most amines, the nitrogen atom is sp3...Ch. 1 - Predict the hybridization and geometry of the...Ch. 1 - Draw orbital pictures of the pi bonding in the...Ch. 1 - Prob. 1.56SPCh. 1 - Prob. 1.57SPCh. 1 - Which of the following compounds show cis-trans...Ch. 1 - Give the relationships between the following pairs...Ch. 1 - Dimethyl sulfoxide (DMSO) has been used as an...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Linoleic acid is an essential fatty acid found in many vegetable oils, such as soy, peanut, and cottonseed. A key structural feature of the molecule is the cis orientation around its two double bonds, where R1 and R2 represent two different groups that form the rest of the molecule. (a) How many different compounds are possible, changing only the cis-trans arrangements around these two double bonds? (b) How many are possible for a similar compound with three double bonds?arrow_forwardsuppose you forget to take into account the presence of unshared pair of electrons on nitrogen in the molecule nh3. what would you then predict for the h-n-h bond angles and geometry of ammonia?arrow_forwardMany reactions involve a change in hybridization of one or more atoms in the starting material. In following reaction, identify the atoms in the organic starting material that change hybridization and indicate the change.arrow_forward
- Acrylic fibers are polymers made from a starting material called acrylonitrile, H2C(CH)CN. In acrylonitrile, a - CN group replaces a hydrogen atom on ethene. Draw the Lewis diagram for this molecule, give the hybridization of each carbon atom, and describe the \pi orbitals and the number of electrons that occupy each one. Draw the three-dimensional structure of the molecule, showing all angles.arrow_forwardWhat are the hybridisations of atoms 1, 2, and 3 in glycine? (Note: lone pairs are NOT shown but may need to be added).arrow_forwardPredict the hybridization, geometry, and bond angles for the carbon atoms in acetylene, C2H2.arrow_forward
- Harry creates a compound that contains only 1 pi (π) bond in total. The central atom has 4s2, 3d10, and 4p5. There are only 3 surrounding atoms with the electron configuration: 1s2 2s2 2p5. What is the formula and VSEPR shape for this compound? Explain how this is possible with respect to hybridized orbitals and how electrons are moved around to create this compound.arrow_forwardThe carbon–carbon bond length in C2H2 is 1.20 Å, that in C2H4 is 1.34 Å, and that in C2H6 is 1.53 Å. Near which of these values would you predict the bond length of C2 to lie? Is the experimentally observed value, 1.31 Å, consistent with your prediction?arrow_forward2a) The molecule ethene (or ethylene), which has the molecular formula C₂H4, contains two carbon atoms with planar geometry. Construct a model of ethene by first connecting two black balls with two springs. Use four short sticks and four yellow balls to complete the structure. Sketch a perspective representation (a three dimensional drawing) of the structure. 2b) What is the C=C-H bond angle in ethene?arrow_forward
- During our lectures on polymeric materials, I used polyethylene as an example because of its very straightforward structure. (a.) Ethylene (C2H4) is a colorless flammable gas. Draw a diagram to show what a single molecule of this gas looks like. Show all bonds with the following symbol (—). Recall that the bonds are highly covalent in nature.arrow_forwardDraw the Lewis Structures for the following molecules and give the hybridization for each carbon atom. a) 2-methyl-1-butanol, CH3CH2CH2(CH3)CH2OH b) Ethyl propanoate, CH3CH2CO2CH2CH3 c) Methyl cyanide (aka acetonitrile), CH3CN d) Isopropyl phenyl ether, (CH3)2CHOC6H5 e) N,N-dimethylpentamide, CH3CH2CH2CH2CON(CH3)2arrow_forwardDimethyl sulfoxide (DMSO) has been used as an anti-inflammatory rub for racehorses. DMSO and acetone appear to have similar structures, but the C“O carbon atom in acetone is planar, while the S“O sulfur atom in DMSO ispyramidal. Draw Lewis structures for DMSO and acetone, predict the hybridizations and explain these observations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY