
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 28GQ
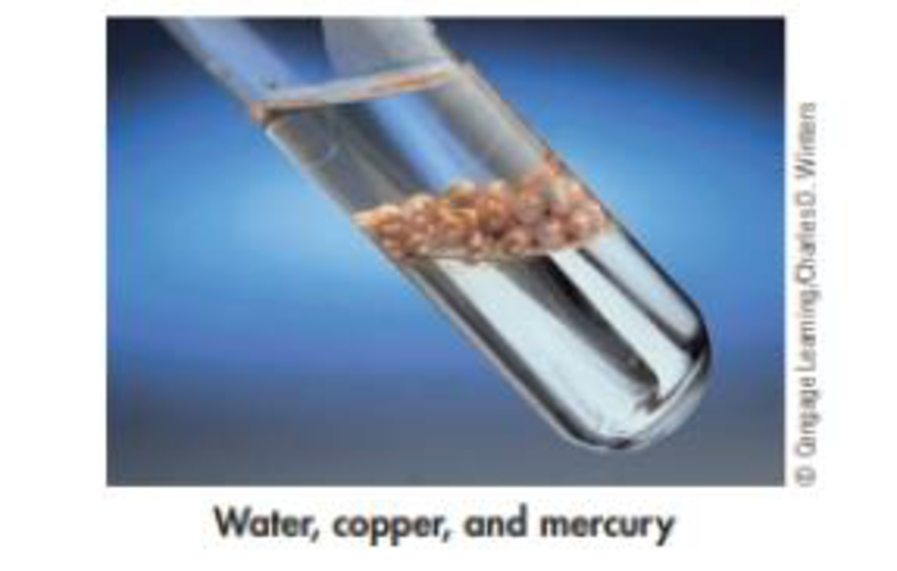
The following photo shows copper balls, immersed in water, floating on top of mercury. What are the liquids and solids in this photo? Which substance is most dense? Which is least dense?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Give an accurate word definition of the term “density”.
Is density a physical property or a chemical property? ___________________________
Is density an intensive property or an extensive property? ___________________________
What information would be needed to calculate the density of a spherical object?
Give an accurate word definition of “specific gravity”.
The volume of a sample of oxygen gas changed from 10 mL to 11 mL as the temperature changed. Is this a chemical or physical change?
A beaker containing a green liquid is left uncovered in a laboratory for one week., After the liquid evaporates, the beaker contains a dry green solid. Was the original liquid in the beaker an element, a compound, or a mixture?
Chapter 1 Solutions
Chemistry & Chemical Reactivity
Ch. 1.3 - Which of the following is a pure substance? (a)...Ch. 1.3 - Which of the following is not characteristic of a...Ch. 1.4 - Prob. 1RCCh. 1.4 - Prob. 2RCCh. 1.5 - Prob. 1RCCh. 1.5 - 2. Which of the following is NOT a...Ch. 1.6 - Which of the following is NOT a physical property?...Ch. 1.6 - 2. A piece of a polypropylene rope (used for water...Ch. 1.6 - Which of the following is an extensive property of...Ch. 1.7 - When camping in the mountains, you boil a pot of...
Ch. 1.8 - Much has been written about CO2.What is its name?Ch. 1.8 - Prob. 2QCh. 1.8 - Prob. 3QCh. 1.8 - The spines of the sea urchin, corals, and...Ch. 1.8 - Which of the following has the highest thermal...Ch. 1.8 - Prob. 2RCCh. 1 - Give the name of each of the following elements:...Ch. 1 - Give the name of each of the following elements:...Ch. 1 - Give the symbol for each of the following...Ch. 1 - Give the symbol for each of the following...Ch. 1 - In each of the following pairs, decide which is an...Ch. 1 - In each of the following pairs, decide which is an...Ch. 1 - In each case, decide if the underlined property is...Ch. 1 - In each case, decide if the change is a chemical...Ch. 1 - Which part of the description of a compound or...Ch. 1 - Which part of the description of a compound or...Ch. 1 - The flashlight in the photo does not use...Ch. 1 - A solar panel is pictured in the photo. When light...Ch. 1 - Determine which of the following represent...Ch. 1 - Prob. 14PSCh. 1 - Prob. 15GQCh. 1 - Iron pyrite (fool's gold, page 11) has a shiny...Ch. 1 - Which observations below describe chemical...Ch. 1 - Which observations below describe chemical...Ch. 1 - The mineral fluorite contains the elements calcium...Ch. 1 - Azurite, a blue, crystalline mineral, is composed...Ch. 1 - You have a solution of NaCI dissolved in water....Ch. 1 - Small chips of iron are mixed with sand (see...Ch. 1 - Identify the following as either physical changes...Ch. 1 - Identify the following as either physical changes...Ch. 1 - In Figure 1.2 you see a piece of salt and a...Ch. 1 - In Figure 1.5 you see macroscopic and particulate...Ch. 1 - Prob. 27GQCh. 1 - The following photo shows copper balls, immersed...Ch. 1 - Categorize each of the following as an element, a...Ch. 1 - Categorize each of the following as an element, a...Ch. 1 - Make a drawing, based on the kinetic-molecular...Ch. 1 - Make a drawing, based on the kinetic-molecular...Ch. 1 - Hexane (C6H14, density = 0.766 g/cm3),...Ch. 1 - You have a sample of a white crystalline substance...Ch. 1 - You can figure out whether a solid floats or sinks...Ch. 1 - You are given a sample of a silvery metal. What...Ch. 1 - Milk in a glass bottle was placed in the freezing...Ch. 1 - Describe an experimental method that can be used...Ch. 1 - Diabetes can alter the density of urine, so urine...Ch. 1 - Prob. 40GQCh. 1 - The following photo shows the element potassium...Ch. 1 - Prob. 42GQCh. 1 - Four balloons are each filled with a different...Ch. 1 - Prob. 44GQCh. 1 - The photo below shows elemental iodine dissolving...Ch. 1 - A few years ago a young chemist in Vienna,...
Additional Science Textbook Solutions
Find more solutions based on key concepts
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Draw a Lewis structure for each covalent molecule. a. HBr b. CH3F c. H2O2 d. N2H4 e. C2H6 f. CH2Cl2
Principles of General, Organic, Biological Chemistry
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of mercury(II) oxide was heated to produce mercury metal and oxygen gas. Then the liquid mercury was cooled to 40C, where it solidified. A glowing wood splint was thrust into the oxygen, and the splint burst into flame. Identify each physical change and each chemical change.arrow_forward1-82 When the astronauts walked on the Moon, they could make giant leaps in spite of their heavy gear. (a) Why were their weights on the Moon so small? (b) Were their masses different on the Moon than on the Earth?arrow_forwardWhich of the following are pure substances and which are mixtures: a table salt; b tap water; c clean, dry air; d steam.arrow_forward
- What is the average density of a single marble shown here? The total mass of the three marbles is 96.5 g. The photograph on the left shows the graduated cylinder filled with 61 mL of water before the marbles were added.arrow_forwardThe following are properties of substances. Decide whether each is a physical property or a chemical property. a Chlorine gas liquefies at 35C under normal pressure. b Hydrogen burns in chlorine gas. c Bromine melts at 7.2C. d Lithium is a soft, silvery-colored metal. e Iron rusts in an atmosphere of moist air.arrow_forwardWhich of the following are the same and which are different? a. a substance and a pure substance b. a heterogeneous mixture and a solution c. a substance and a mixture d. a homogeneous mixture and a solutionarrow_forward
- 1.51 A person measures 173 cm in height. What is this height in meters? Feet and inches?arrow_forwardAzurite, a blue, crystalline mineral, is composed of copper, carbon, and oxygen. (a) What are the symbols of the three elements that combine to make the mineral azurite? (b) Based on the photo, describe some of the physical properties of the elements and the mineral. Are any the same? Are any properties different?arrow_forwardThe cup is a measure of volume widely used in cook-books. One cup is equivalent to 225 mL. What is the density of clover honey (in grams per milliliter) if three quarters of a cup has a mass of 252 g?arrow_forward
- 1-35 You are taken for a helicopter ride in Hawaii from Kona (sea level) to the top of the volcano Mauna Kea. Which property of your body would change during the helicopter ride? (a) height (b) weight (c) volume (d) massarrow_forwardA bottle of sports drink is homogeneous or heterogenous ? B) well water is a pure substance of a mixturearrow_forward1.14 Classify each of the following as a pure substance or amixture. If a mixture, indicate whether it is homogeneous orheterogeneous: (a) air, (b) tomato juice, (c) iodine crystals,(d) sand.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY