
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
4th Edition
ISBN: 9781119761105
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1, Problem 55PP
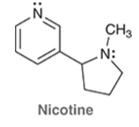
Nicotine is an addictive substance found in tobacco. Identify the hybridization state and geometry of each the nitrogen atoms in nicotine:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify the hybridization state, molecular geometry and approximate bond angle at the indicatednitrogen atom in the following
compound.
O sp²,bent, 109°
O sp²,bent, 120°
O sp3, tetrahedral, 109.5°
O sp3, trigonal pyramidal, ~109.5°
O sp2, trigonal pyramidal, 120°
CH3 HH CH₂.
H₂=C₂C=C₂C₂O₂H
N
OU!
H³N¬C-C¬-H
H
HH
.0.
Predict the geometry, hybridization, and bond angles for the central atoms in the following compound.
Two important industrial chemicals, ethene, C2H4, and propene, C3H6, are produced by the steam (or thermal) cracking process: For each of the four carbon compounds, do the following:
Draw a Lewis structure.
Predict the geometry about the carbon atom.
Determine the hybridization of each type of carbon atom.
Chapter 1 Solutions
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
Ch. 1.2 - Prob. 1LTSCh. 1.2 - Prob. 2ATSCh. 1.2 - Prob. 2LTSCh. 1.3 - Prob. 3LTSCh. 1.3 - Prob. 4PTSCh. 1.3 - Prob. 5PTSCh. 1.4 - Prob. 4LTSCh. 1.4 - Prob. 7PTSCh. 1.4 - Prob. 8PTSCh. 1.4 - Prob. 9ATS
Ch. 1.5 - Prob. 5LTSCh. 1.5 - Prob. 10PTSCh. 1.5 - Prob. 11ATSCh. 1.5 - Prob. 12ATSCh. 1.6 - Prob. 6LTSCh. 1.6 - Prob. 14ATSCh. 1.7 - Prob. 7LTSCh. 1.7 - Prob. 17ATSCh. 1.10 - Prob. 18CCCh. 1.10 - Prob. 20CCCh. 1.10 - Prob. 8LTSCh. 1.10 - Prob. 21PTSCh. 1.10 - Nemotin is a compound that was first isolated from...Ch. 1.10 - Prob. 23CCCh. 1.11 - Prob. 9LTSCh. 1.11 - Prob. 24PTSCh. 1.11 - Prob. 25PTSCh. 1.11 - Prob. 26PTSCh. 1.11 - Prob. 27ATSCh. 1.12 - Prob. 10LTSCh. 1.12 - Prob. 29ATSCh. 1.13 - Prob. 11LTSCh. 1.13 - Prob. 31ATSCh. 1 - Prob. 32PPCh. 1 - Prob. 33PPCh. 1 - Prob. 34PPCh. 1 - Prob. 35PPCh. 1 - Prob. 36PPCh. 1 - Prob. 37PPCh. 1 - Prob. 38PPCh. 1 - Prob. 39PPCh. 1 - Prob. 40PPCh. 1 - Prob. 41PPCh. 1 - Prob. 42PPCh. 1 - Prob. 44PPCh. 1 - Prob. 45PPCh. 1 - Prob. 46PPCh. 1 - Prob. 47PPCh. 1 - Prob. 48PPCh. 1 - Prob. 49PPCh. 1 - Prob. 50PPCh. 1 - Prob. 51PPCh. 1 - Prob. 52PPCh. 1 - Prob. 53PPCh. 1 - Prob. 54PPCh. 1 - Nicotine is an addictive substance found in...Ch. 1 - Prob. 56PPCh. 1 - Prob. 57PPCh. 1 - Prob. 59PPCh. 1 - Prob. 63ASPCh. 1 - Prob. 64ASPCh. 1 - Prob. 66ASPCh. 1 - Prob. 69ASPCh. 1 - Prob. 71ASPCh. 1 - Prob. 72ASPCh. 1 - Prob. 75IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (benzene rings are frequently pictured as hexagons, without the letter for the carbon atom at each vertex). Note that the drawings do not necessarily depict the bond angles correctly.arrow_forwardThere are two compounds with the molecular formula HN3. One is called hydrogen azide; the other is cyclotriazene. (a) Write the Lewis structure for each compound. (b) Designate the hybridization of each nitrogen in hydrogen azide. (c) What is the hybridization of each nitrogen in cyclotriazene? (d) How many sigma bonds are in hydrogen azide? In cyclotriazene? (e) How many pi bonds are in hydrogen azide? In cyclotriazene? (f) Give approximate values for the N-to-N-to-N bond angles in each molecule.arrow_forwardThe hybridization of the two carbon atoms differs in an acetic acid, CH3COOH, molecule. (a) Designate the correct hybridization for each carbon atom in this molecule. (b) What is the approximate bond angle around each carbon?arrow_forward
- Identify the type of hybridization, approximate bond angles for the N, C, and O atoms, and shortest carbon-to-oxygen bond length in alanine, an amino acid, whose Lewis structure isarrow_forwardMethylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is (a) Give values for the three bond angles indicated. (b) Indicate the most polar bond in the molecule. (c) Circle the shortest carbon-oxygen bond. (d) Circle the shortest carbon-carbon bond.arrow_forwardMolecule G Lewis structure Hybridization PFs Number of o and n bonds Number of valence electrons Number of bonded atoms Molecular shape on central atom Number of lone pairs on central atom Bonded-atom lone- Polarity pair arrangement Central atom Bond angles Bond order steric number P-F: Molecule H Lewis structure Hybridization TeF6 Number of valence Number of o and n electrons bonds 0: Number of bonded atoms Molecular shape on central atom Number of lone pairs Bonded-atom lone- Polarity on central atom pair arrangement Central atom Bond angles Bond order steric number Тe-F:arrow_forward
- Harry creates a compound that contains only 1 pi (π) bond in total. The central atom has 4s2, 3d10, and 4p5. There are only 3 surrounding atoms with the electron configuration: 1s2 2s2 2p5. What is the formula and VSEPR shape for this compound? Explain how this is possible with respect to hybridized orbitals and how electrons are moved around to create this compound.arrow_forward7. What is the hybridization state of the indicated atoms in the following compound?arrow_forwardcarbon is the master of hybridization, name 3 different compounds that showcase 3 different hybridization variants in the carbon atom. Include compond names, lewis diagram and the hybridization of the carbon atom. For each of the following compounds draw the lewis diagram. Include VSEPR family, the VSEPR shape? Determine the color of light emitted from an electron in a hydrogen atom that falls from n = 6 to n = 2.arrow_forward
- Identify the hybridization state, molecular geometry and approximate bond angle around the carbon atomfor the molecule shown in box below. sp², tetrahedral, 109° sp², trigonal planar, 120° sp³, tetrahedral, 109.5° sp³, trigonal pyramidal, <109.5° O sp², trigonal pyramidal, 180° HCOOHarrow_forwardIon E Lewis structure Hybridization [H;O]* Number of valence Number of o and n electrons bonds O: Number of bonded atoms Molecular shape on central atom Number of lone pairs on central atom Bonded-atom lone- Polarity pair arrangement Central atom Bond angles Bond order steric number O-H: Molecule F Lewis structure Hybridization BRNS Number of valence Number of o and n electrons bonds 0: Number of bonded atoms Molecular shape on central atom Bonded-atom lone- Number of lone pairs on central atom Polarity pair arrangement Central atom Bond angles Bond order steric number N-Br: N-S:arrow_forwardWhich of the following can form hybrid orbitals? Group of answer choices Only sigma bond electrons Only lone pair electrons Pi and lone pair electrons Only Pi bond electrons Sigma and pi bond electrons Sigma and lone pair electrons Sigma, pi and lone pair electronsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY