
Interpretation: The number of carbon atoms and
-bonds present in the following compound should be identified:
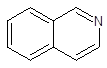
Concept Introduction: A method used to represent molecular structures of compounds is said to be bond line notation. In this notation, a line depicts a bond between two atoms and are drawn in a zigzag format. Atoms other than carbon and hydrogens are specifically depicted in this notation. It is assumed that carbon atoms are bonded to enough hydrogen atoms that are required to complete the octet.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY LL BUNDLE
- 3-25 Why are carbon and silicon reluctant to form ionic bonds?arrow_forwardAre the bonds in each of the following substances ionic,nonpolar covalent, or polar covalent? Arrange the substanceswith polar covalent bonds in order of increasing bond polarity:(a) KCl (b) P₄(c) BF₃(d) SO₂(e) Br₂(f) NOarrow_forwardDraw a Lewis Structure for each of the following species and assign formal charge where appropriate. Using electronegative values from the period table that was provided identify polar covalent bonds and label the atoms δ+ and δ−. For each of the molecules indicate whether or not it has a dipole moment. (a)CH5N (b) HCN (c) H2CO (d) CH3NC(e) CH3SOCH3 (f) H6BNarrow_forward
- Oxalic acid, H2C2O4, a poisonous colorless solid, is found in some vegetables such as spinach and rhubarb. It is present in concentrations well below the toxic limit, so you can't use this as a reason to refuse a helping of spinach. The order of atoms in a molecule of oxalic acid is HO2CCO2H. (a) How many unshared pairs of electrons are on each of the carbon atoms? (b) How many unshared pairs of electrons are on each of the oxygen atoms?arrow_forwardSemustine is a chemotherapy drug, and exerts it's anticancer activity by damaging the DNA of the cancer cells. Predict the approximate bond angle, (c) in Semustine and the molecular geometry of the indicated oxygen (a) and nitrogen (b) atoms. (a) NH (c) CH (b) Semustine NINAarrow_forwardDraw Lewis structures for the following compounds. Remember to enclose ions in square brackets. On your answer sheet, state the number of valence electrons in each compound. (a) NaCN (b) CH 3 Br (c) Ca(OCl) 2arrow_forward
- From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity:(a) As, H, N, P, Sb(b) Cl, H, P, S, Si(c) Br, Cl, Ge, H, Sr(d) Ca, H, K, N, Si(e) Cl, Cs, Ge, H, Srarrow_forwardJudging from their relative positions in the Periodic Table, which atom in each set is more electronegative? (a) Carbon or nitrogen (b) Chlorine or bromine (c) Oxygen or sulfurarrow_forwardDraw a Lewis structure for each of the following molecule: (a) chlorodifluoromethane, CHCIF2 (b) propanoic acid C2H5CO2H (basic structure pictured below) (c) acetonitrile, CH3CN ( the framework is H3C-C-N) (d) allene, H2CCCH2arrow_forward
- Arrange the bonds in each of the following sets in order of increasing polarity: (a) C-F, O-F, Ве-F (b) O-CI, S-Br, C-P (c) С-S, B-F, N-0arrow_forwardUsing the symbols 8- and &+, indicate the direction of polarity, if any, in each covalent bond. (а) С—СІ (b) S-H (c) C-S (d) Р—Нarrow_forwardAre the bonds in each of the following substances ionic,nonpolar covalent, or polar covalent? Arrange the substanceswith polar covalent bonds in order of increasing bond polarity:(a) S₈(b) RbCl (c) PF₃(d) SCl₂(e) F₂(f) SF₂arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




