
a.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
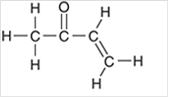
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form
b.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
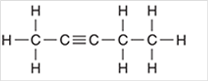
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
c.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
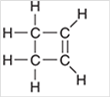
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
d.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
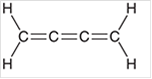
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
e.
Interpretation: The hybridization state for each carbon atom in the following compound should be identified:
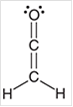
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form chemical bond is said to be hybridization.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY LL BUNDLE
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





