
Define these terms: system, surroundings, thermal energy, chemical energy.
Interpretation: The terms system, surroundings, thermal energy and chemical energy has to be defined.
Concept Introduction:
The terms system, surroundings, thermal energy and chemical energy are involved in thermodynamic process.
System can be defined as portion of universe and surrounding can be defined as rest of the universe other than the system.
Thermal energy can be defined as internal energy that is seen in the system because of its temperature.
Chemical energy can be defined as the energy that is seen in the chemical bonds of atoms and molecules. The chemical energy occurs as energy released during a chemical reaction
Answer to Problem 10.1QP
System can be defined as portion of universe.
Surrounding can be defined as rest of universe other than the system.
Thermal energy can be defined as internal energy that is seen in the system because of its temperature.
Chemical energy can be defined as the energy that is seen in the chemical bonds of atoms and molecules. The chemical energy occurs as energy released during a chemical reaction
Explanation of Solution
System can be defined as portion of universe. The physical and chemical changes of substance generally constitute a system.
There are types of system in thermodynamics namely,
- 1. Open system
- 2. Closed system
- 3. Isolated system
Open system: The free exchange of matter and energy with its surroundings is called as open system. The exchange of matter in open system takes place either by addition of matter or removal of matter. The exchange of energy is much more complicated than exchange of heat. The exchange of energy takes place through heat and through work.
The figure below shows the transfer of energy and matter.
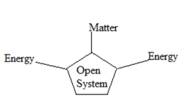
Figure 1
Closed system: The exchange of energy with its surroundings and not matter is called as closed system. The transfer of energy is similar to that of open system
The figure below shows the transfer of energy in close system
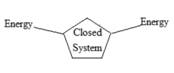
Figure 2
Isolated system: Either exchange of energy or matter takes place with the surroundings is called isolated system.
To define surroundings
Surrounding can be defined as rest of universe other than the system.
Explanation:
Any part of the universe other than the system is called as surroundings.
Consider, the example of acid-base neutralization reaction,
The reactants
To define thermal energy
Thermal energy can be defined as internal energy that is seen in the system because of its temperature.
Explanation:
Thermal energy can be defined as internal energy that is seen in the system because of its temperature. Thermal energy deals the unsystematic motion of atoms and molecules.
Its type of kinetic energy that is due to motion. Thermal energy results in substance possessing an internal temperature, which can be measured.
Consider, the example below
Thermometer having degrees in Celsius or Fahrenheit, the particles move faster within an object or system, higher the temperature is recorded.
To define chemical energy
Chemical energy can be defined as the energy that is seen in the chemical bonds of atoms and molecules. The chemical energy occurs as energy released during a chemical reaction.
Explanation:
The form of energy that is stored in chemical bonds of atoms and molecules are called as chemical energy. The chemical energy occurs as energy released during a chemical reaction called as exothermic energy
Examples of matter containing chemical energy are,
- 1) Coal- Chemical energy is converted into light and heat.
- 2) Wood- Chemical energy is converted into light and heat. Etc
Want to see more full solutions like this?
Chapter 10 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- Explain the difference between heat capacity and specific heat of a substance.arrow_forward9.30 For the example of shallow water and sandy beaches, which material has a larger heat capacity or specific heat? How does a hot day at the beach provide evidence for your answer?arrow_forwardThe temperature of the cooling water as it leaves the hot engine of an automobile is 240 F. After it passes through the radiator it has a temperature of 175 F. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184 J/g oC.arrow_forward
- Define heat. What are its units? How does it differ from energy?arrow_forwardDetermine whether the statements given below are true or false. Consider an endothermic process taking place in a beaker at room temperature. (a) Heat flows from the surroundings to the system. (b) The beaker is cold to the touch. (c) The pressure of the system decreases. (d) The value of q for the system is positive.arrow_forwardIn the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s)+3CO(g)2Fe(s)+3CO2(g)H=24.8kJ The enthalpy change for the combustion of carbon monoxide is 2CO(g)+O2(g)2CO2(g)H=566kJ Use this information to calculate the enthalpy change for the equation 4Fe(s)+3O2(g)2Fe2O3(s)H=?arrow_forward
- Explain why absolute enthalpies and energies cannot be measured, and only changes can be determined.arrow_forwardA piece of chocolate cake contains about 400 calories. A nutritional calorie is equal to 1000 calories (thermochemical calories), which is equal to 4.184 kJ. How many 8-in-high steps must a 180-lb man climb to expend the 400 Cal from the piece of cake? See Exercise 28 for the formula for potential energy.arrow_forwardIf 125 J of heat energy is applied to a block of silver weighing 29.3 g, by how many degrees will the temperature of the silver increase? (See Table 10.1.)arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





