
(a)
Interpretation:
The product expected when
Concept introduction:
Alcohols undergo nucleophilic substitution reaction in the presence of halogen acids. The hydroxide group leaves after protonation and halogen group comes in via
Answer to Problem 10.40AP
The product obtained on the reaction of
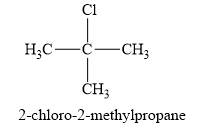
Explanation of Solution
The product obtained on the reaction of
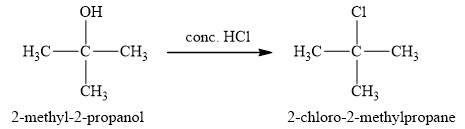
Figure 1
The
The product obtained on the reaction of
(b)
Interpretation:
The product expected when
Concept introduction:
Primary and secondary alcohols can be oxidized into
Answer to Problem 10.40AP
No product is obtained on the reaction of
Explanation of Solution
The product obtained on the reaction of
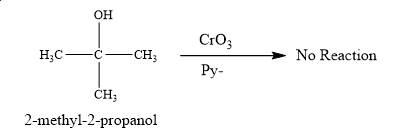
Figure 2
No reaction takes place between
No product is obtained on the reaction of
(c)
Interpretation:
The product expected when
Concept introduction:
An
Answer to Problem 10.40AP
The product obtained on the reaction of
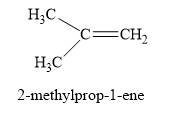
Explanation of Solution
The product obtained on the reaction of
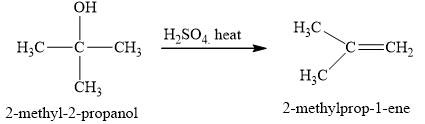
Figure 3
The alcohol
The product obtained on the reaction of
(d)
Interpretation:
The product expected when
Concept introduction:
Free radical reaction are the reaction in which the bond between the molecule is broken homolytically due to the presence of light energy. These kind of reactions are generally obtained with alkenes when reacted with halogen in the presence of light or peroxides.
Answer to Problem 10.40AP
No product is obtained when
Explanation of Solution
No reaction product is obtained on the reaction of
No product is obtained when
(e)
Interpretation:
The product expected when
Concept introduction:
Acid-base reaction are among the fastest reaction in the chemistry. Acids and bases reacts vigorously generating heat and water normally. Metals are basic in nature due to the presence of free electrons to donate. Alcohols are both acidic and basic in nature.
Answer to Problem 10.40AP
The product obtained on the reaction of
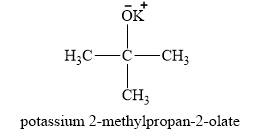
Explanation of Solution
The product obtained on the reaction of
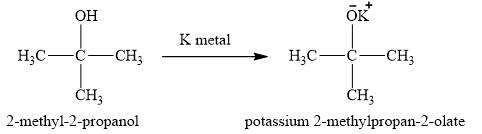
Figure 4
The alcohol
The product obtained on the reaction of
(f)
Interpretation:
The product expected when
Concept introduction:
The hydroxide group in alcohols is not a good leaving group that can perform a nucleophilic substitution reaction on alcohols to produce more compounds. Hydroxide group is made a good leaving group with the help of some compounds like methanesulfonyl chloride and p-toluenesulfonyl chloride.
Answer to Problem 10.40AP
The product obtained on the reaction of
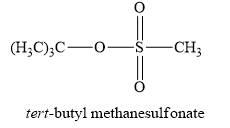
Explanation of Solution
The product obtained on the reaction of
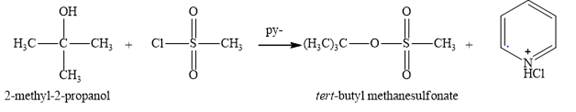
Figure 5
The reaction of an alcohol with sulfonate derivatives such as methanesulfonyl chloride and p-toluenesulfonyl chloride are done to make hydroxide group and good leaving group. Pyridine being more basic takes up the proton of the hydroxide group make it a good nucleophile at first. This nucleophiles then substitute the chloride group of methanesulfonyl chloride leading to the formation of an ester. Same happens here in this reaction
The product obtained on the reaction of
(g)
Interpretation:
The product expected when the product of part (f) is reacted with
Concept introduction:
An
Answer to Problem 10.40AP
The product expected when the product of part (f) is reacted with
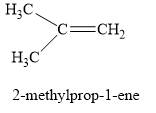
Explanation of Solution
The product obtained in part (f) is
The product obtained on the reaction of
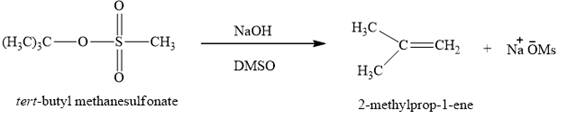
Figure 6
The
The product expected when the product of part (f) is reacted with
(h)
Interpretation:
The product expected when the product of part (e) is reacted with the product of part (a) is to be stated.
Concept introduction:
An
Answer to Problem 10.40AP
The products obtained on the reaction of
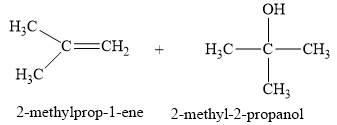
Explanation of Solution
The product of part (a) is
The products obtained on the reaction of
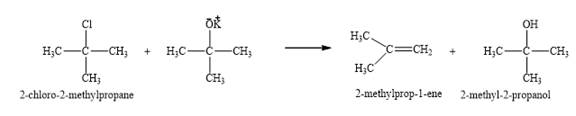
Figure 7
The reaction undergoes via
The products obtained on the reaction of
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





