
(a)
Interpretation:
Degree of freedom for
Concept Introduction:
The Gibbs phase rule provides the relation between number of chemically independent component and the number of independent phases for a given system. When temperature and pressure of the system can be changed, it is defined as:
Here,
Answer to Problem 10.41P
For temperature
For temperature
Explanation of Solution
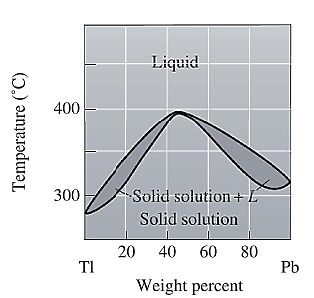
The phase diagram given for the Tl-Pb solution is given as:
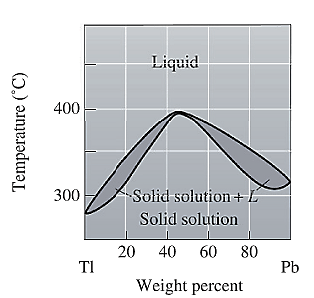
For
For the given system, the pressure is assumed to be constant. Thus, the formula used to calculate degree of freedom is:
For temperature
Apply equation (1) and calculate degree of freedom as:
For temperature
Apply equation (1) and calculate degree of freedom as:
(b)
Interpretation:
Degree of freedom for
Concept Introduction:
The Gibbs phase rule provides the relation between number of chemically independent component and the number of independent phases for a given system. When temperature and pressure of the system can be changed, it is defined as:
Here,
Answer to Problem 10.41P
For temperature
For temperature
Explanation of Solution
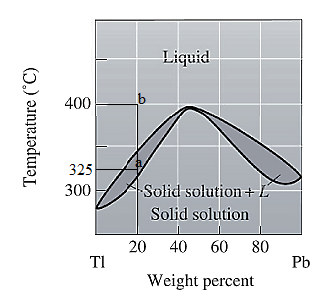
The phase diagram given for the Tl-Pb solution is given as:
For
For the given system, the pressure is assumed to be constant. Thus, the formula used to calculate degree of freedom is:
For temperature
Apply equation (1) and calculate degree of freedom as:
For temperature
Apply equation (1) and calculate degree of freedom as:
(c)
Interpretation:
Degree of freedom for
Concept Introduction:
The Gibbs phase rule provides the relation between number of chemically independent component and the number of independent phases for a given system. When temperature and pressure of the system can be changed, it is defined as:
Here,
Answer to Problem 10.41P
For temperature
For temperature
Explanation of Solution
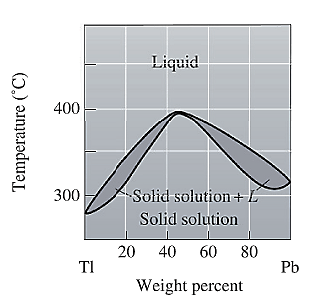
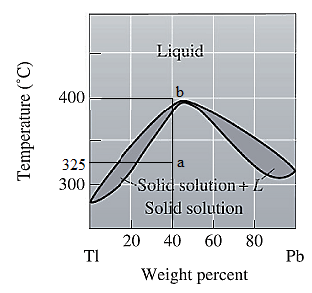
The phase diagram given for the Tl-Pb solution is given as:
For
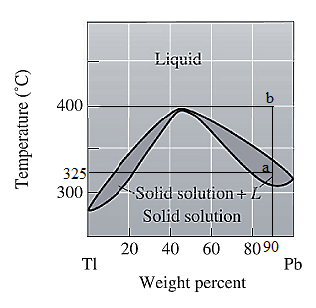
For the given system, the pressure is assumed to be constant. Thus, the formula used to calculate degree of freedom is:
For temperature
Apply equation (1) and calculate degree of freedom as:
For temperature
Apply equation (1) and calculate degree of freedom as:
Want to see more full solutions like this?
Chapter 10 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





