
(a)
Interpretation:
The composition of the solid phase and the liquid phase in wt% and at% are to be calculated for
Concept Introduction:
On the temperature-composition graph of analloy, the curve above which the alloy exist in the liquid phase is the liquidus curve. The temperature at this curve is the maximum temperature at which the crystals in the alloy can coexist with its melt in the
Solidus curve is the locus of the temperature on the temperature composition graph of analloy, beyond which the alloy is completely in solid phase.
Between the solidus and liquidus curve, the alloy exits in a slurry form in which there is both crystals as well as alloy melt.
Solidus temperature is always less than or equal to the liquidus temperature.
The formula to calculate the at% from the wt% for an alloy containing phases
Here,
Answer to Problem 10.54P
Composition of the liquid phase in at% is
Composition of the liquid phase in wt% is
Composition of the solid phase in at% is
Composition of the solid phase in wt% is
Explanation of Solution
The phase diagram for Nb-W alloy is given as:
Now, draw a straight line from temperature
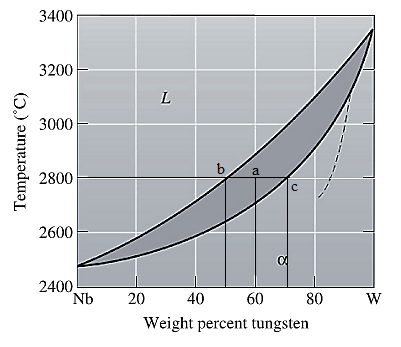
Here, point 'a' represents
Molecular weight of Nb and W are
Use equation (1) to convert wt% to at% for liquid phase as:
Again, use equation (1) to convert wt% to at% for solid phase as:
(b)
Interpretation:
The amount of each phase present in
Concept Introduction:
A matter can exist in different physical forms such as sold, liquid, gas, and plasma. These distinct physical forms are known as a Phase.
A phase has uniform physical and chemical properties and is bounded by a surface due to which two phases can be
The formula to calculate the wt% from the at% for an alloy containing phases
Here,
Amount of each phase in wt% is calculated using lever rule. At a particular temperature and alloy composition, a tie line is drawn on the phase diagram of the alloy between the solidus and liquidus curve. Then the portion of the lever opposite to the phase whose amount is to be calculated is considered in the formula used as:
Answer to Problem 10.54P
Amount of liquid phase in at% is
Amount of liquid phase in wt% is
Amount of solid phase in at% is
Amount of solid phase in wt% is
Explanation of Solution
The phase diagram for Nb-W alloy is given as:
Now, draw a straight line from temperature
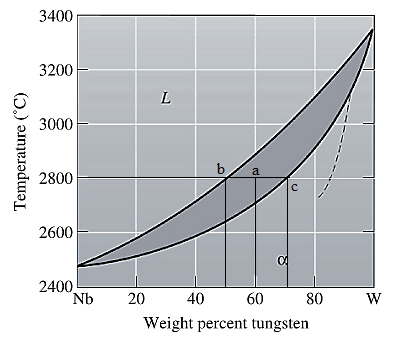
Here, point 'a' represents
To calculate amount of liquid phase, lever 'ac' will be used and to calculate amount of solid phase, lever 'ba' will be used. Use equation (3) to calculate the amount of each phase as:
To calculate the amount of liquid and solid phases in at%, first convert the original wt% of W in at% using equation (1) and molecular weights of Nb and W as:
To apply the lever rule, use the corresponding at% for the liquid and solid phases as calculated in part (a) as:
Apply lever rule as:
(c)
Interpretation:
The amount of each phase is to be calculated in vol%.
Concept Introduction:
The formula to convert wt% to vol% using density
Answer to Problem 10.54P
The amount of liquid phase in Vol% is
The amount of solid phase in Vol% is
Explanation of Solution
Given information:
An alloy containing
From part (b), the amount of liquid and solid phases in wt% is calculated as:
Use equation (4) along with the given densities of the phases to calculate the vol% as:
Want to see more full solutions like this?
Chapter 10 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





