
Concept explainers
(a)
Interpretation:
The given reaction is to be completed and explained to give the principal products.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
The
Answer to Problem 10.59AP
The complete reaction is shown below.
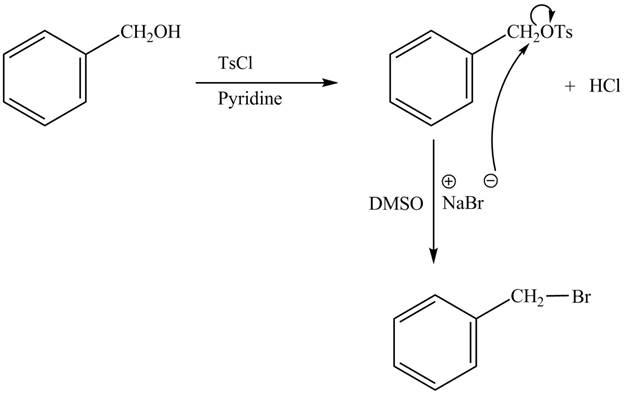
The tosyl chloride is used to make the hydroxide group a good leaving group by replacing its hydrogen with tosyl group. The
Explanation of Solution
The given reaction is shown below.
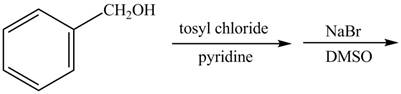
Figure 1
The complete reaction with the products is shown below.
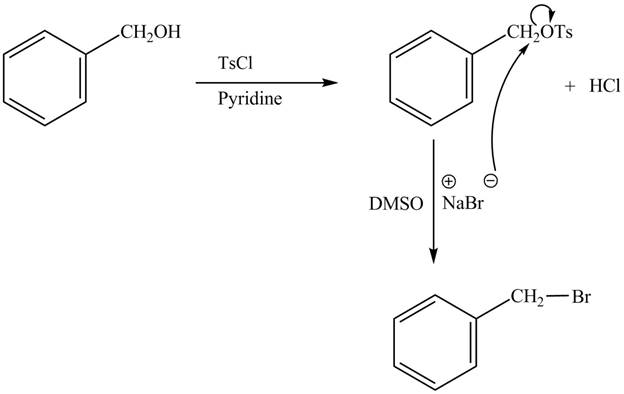
Figure 2
The reaction of the alcohols with tosyl chloride is the reaction to make the hydroxide group a good leaving group. The hydrogen is replaced by the tosyl group. The ![]() to give the halide. The product thus obtained in the end is benzyl bromide.
to give the halide. The product thus obtained in the end is benzyl bromide.
The completed reaction is shown in Figure 2.
(b)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
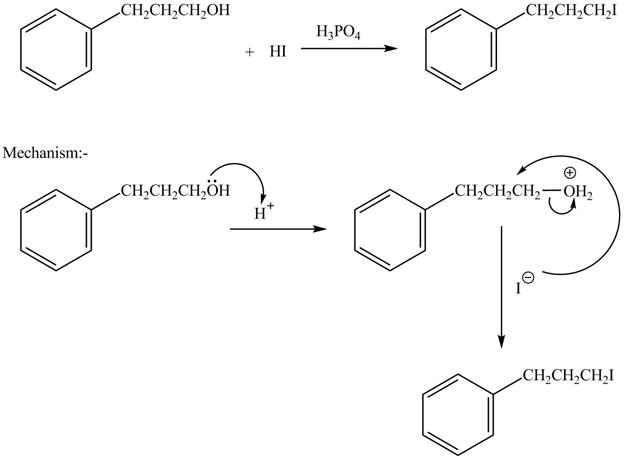
The acid is used to make the hydroxide group a good leaving group. The iodide group than substitutes the protonated hydroxide group to give halide product.
Explanation of Solution
The given reaction is shown below.
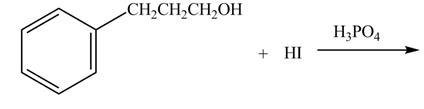
Figure 3
The complete reaction with the products is shown below.

Figure 4
The hydroxide group in alcohols is not a good leaving group in order to perform a nucleophilic substitution reaction on alcohols to produce more compounds. Hydroxide group is made a good leaving group by protonating the hydroxide group in the first step. After then the iodide ion attacks and eliminates protonated hydroxide group to halide product.
The completed reaction is shown in Figure 4.
(c)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
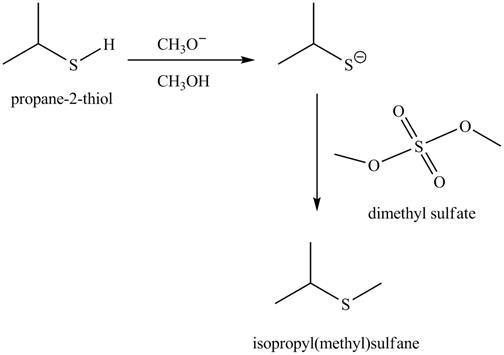
The acid-base reaction between the thiol group and methoxide ion takes place first to give sulfide ion. The sulfide ion then reacts with methylating agent dimethyl sulfate to give the methylated product isopropyl(methyl) sulfane.
Explanation of Solution
The given reaction is shown below.
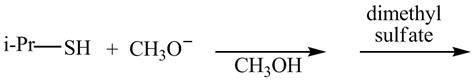
Figure 5
The complete reaction with the products is shown below.
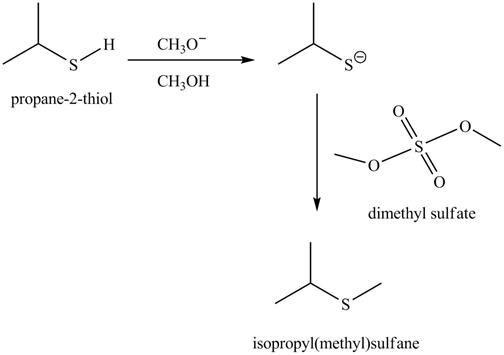
Figure 6
The methoxide ion acts as a base and takes away the hydrogen of the thiol group of
The completed reaction is shown in Figure 6.
(d)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
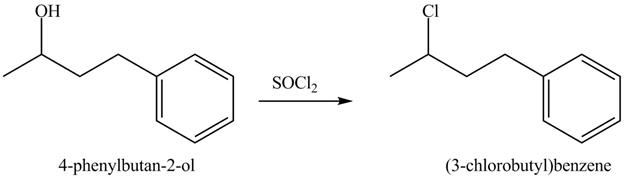
This is an
Explanation of Solution
The given reaction is shown below.
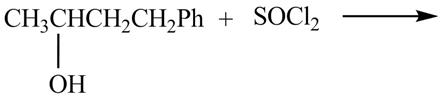
Figure 7
The complete reaction with the products is shown below.

Figure 8
The reaction of alcohols with thionyl chloride is a
The completed reaction is shown in Figure 8.
(e)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
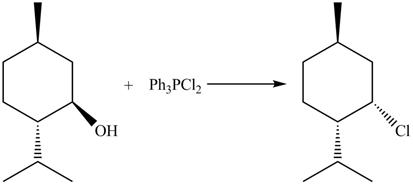
This is an
Explanation of Solution
The given reaction is shown below.
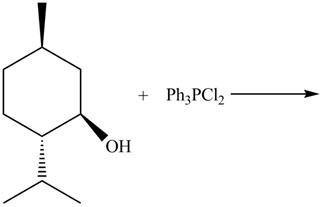
Figure 9
The complete reaction with the products is shown below.
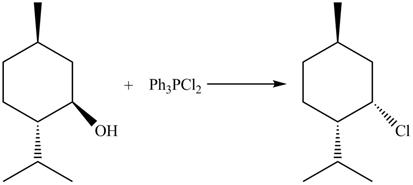
Figure 10
The reaction of alcohols with triphenylphosphine dichloride is a
The completed reaction is shown in Figure 10.
(f)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
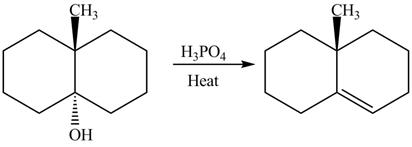
The reaction between an alcohol and acid with heating undergoes dehydration reaction to give alkene as a product.
Explanation of Solution
The given reaction is shown below.
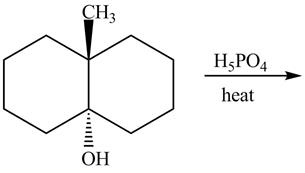
Figure 11
The complete reaction with the products is shown below.

Figure 12
The reaction of alcohols with acids and heat is an
The completed reaction is shown in Figure 12.
(g)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
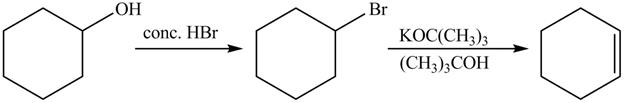
The first reaction is the nucleophilic substitution reaction of hydroxide group by the bromide ion. The second reaction is the elimination reaction in which strong base
Explanation of Solution
The given reaction is shown below.
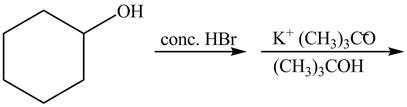
Figure 13
The complete reaction with the products is shown below.

Figure 14
The first step of the reaction is a
The completed reaction is shown in Figure 14.
(h)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
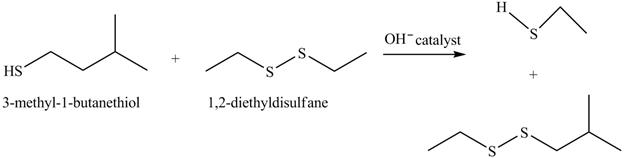
The acid-base reaction between the thiol group and hydroxide ion takes place first to give sulfide ion. The sulfide ion then reacts with diethyl sulfane to give a mixture of thiol and disulfide.
Explanation of Solution
The given reaction is shown below.
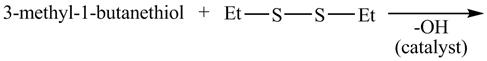
Figure 15
The complete reaction with the products is shown below.
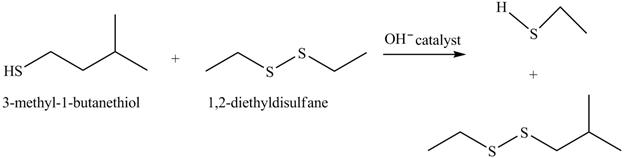
Figure 16
The hydroxide ion acts as a base and takes away the hydrogen of the thiol group of
The completed reaction is shown in Figure 16.
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Compound A is an alcohol that undergoes oxidation to produce compound B.Compound B is a ketone that gives positive triiodomethane reaction. Compound B isthen reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueousacid to form compound C. Compound C has the molecular formula of C9H12O. Deducethe structure for compound A, B and C. PLEASE PROVIDE CLEAR DRAWINGS AND EXPLANATIONSarrow_forwardCompound A is a branched-chain alcohol that undergoes oxidation to produce compound B. Compound B is a ketone that gives positive triiodomethane reaction. Compound B is then reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueous acid to form compound C. Compound C has the molecular formula of C11H16O (i) Deduce the structure for compound A, B and C. (ii) State the observation when compound C is added with acidified potassium dichromate(VI).arrow_forwardA compound A, C7H12, was found to be optically active. On catalytic reduction over platinum catalyst, 2 equivalents of hydrogen were absorbed, yielding compound B, C7H16. On ozonolysis of A, two fragments were obtained. One fragment was identified as acetic acid. The other fragment, compound C, was an optically active carboxylic acid, C5H10O2. Write the reactions and draw structures for A, B and C.arrow_forward
- Name, draw and describe the organic product of the reaction between 2-methylbut-1-ene and H2O in the presence of H2SO4 and provide a clear rationale as to why this is the major product of the reaction and the minor product of the reaction.arrow_forwardGive the structural chemistry of active methylene, classify it asradical/intermediate/stable organic compound, reagent of its production and role in alkylationarrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
- Compound A, C3H7Br, does not react with cold dilute potassium permanganate solution. Upon treatment with potassium hydroxide in ethanol, A gives only product B, C3H6. Unlike A, B decolourises potassium permanganate solution. Ozonolysis of Bgives C, C2H4O, and D, CH2O. Suggest the structural formulae of A, B, C and D.Write the equations for all the reactions involved.arrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent ofH2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragmentis propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are thestructures of A, B, and C? Write all reactions and show your reasoning.arrow_forwardCompound A(C10H12O)gives off oxygen on treatment with sodium metal and also decolorizes Br2 in CCl4 to give organic compound B. Compound A on treatment with I2 in NaOH gives iodoform and salt C which after acidification gives a white solid D(C7H6O2). Using knowledge of organic chemistry identify structures A,B,C and Darrow_forward
- Compound A (C5H8) readily reacts with bromine (Br2) at room temperature to discharge the purple colour of bromine and form Compound B (C5H8Br2). When Compound A is treated with H2 in the presence of a transition metal catalyst, it is converted to compound C (C5H10). When treated with HCl, compound A is converted to compound D (C5H9Cl). B, Cand D are saturated compounds. Given this information, propose structural formulas for compounds A, B, C, and D.arrow_forwardGiven that naphthalene is insoluble in water and sublimes easily upon heating, describe two methods by which you could separate naphthalene and NaCL.arrow_forwardTwo moles of organic compound ‘A’ on treatment with a strong base gives two compounds ‘B’ and ‘C’. Compound ‘B’ on dehydrogenation with Cu gives ‘A’ while acidification of ‘C’ yields carboxylic acid ‘D’ with molecular formula of CH2O2. Identify the compounds A, B, C and D and write all chemical reactions involved.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





