
(a)
Interpretation:
The symmetry elements and point group of staggered
Concept introduction:
A symmetry operation is defined as an action on an object to reproduce an arrangement that is identical to its original spatial arrangement. The spatial arrangement of the object remains identical after a symmetry operation. The point of reference through which a symmetry operation takes place is termed as a symmetry element.
(a)
Answer to Problem 10A.1P
The symmetry elements of staggered
The staggered
Explanation of Solution
The staggered form of ethane
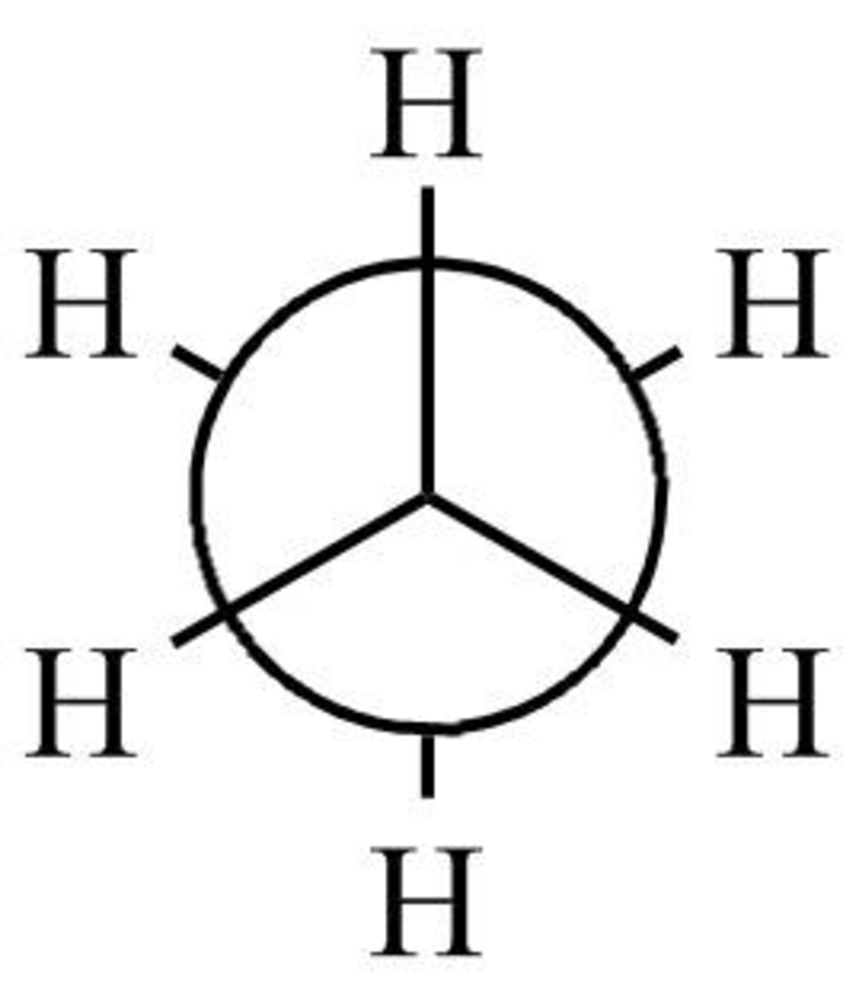
Figure 1
The staggered
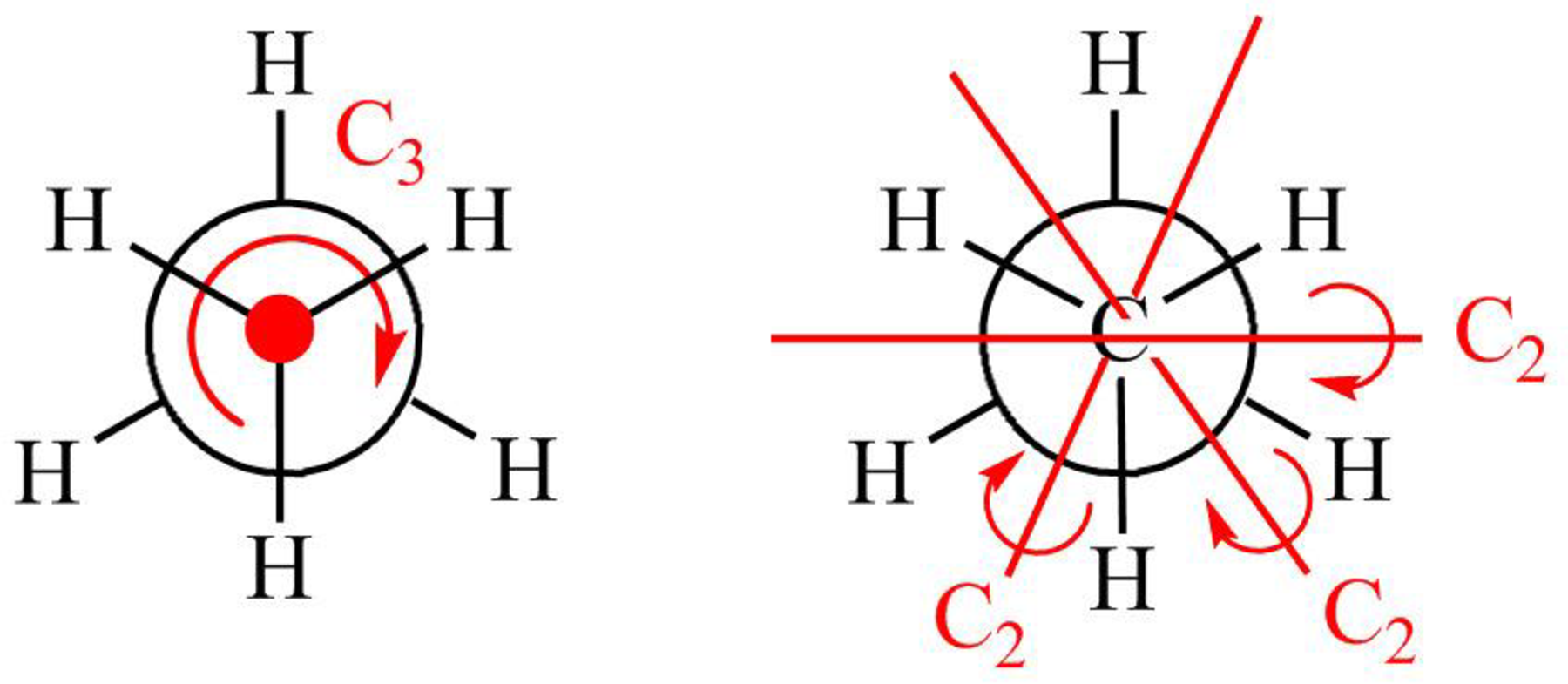
Figure 2
The symmetry elements of staggered
The molecules which have point group
The molecules which do not have any reflective symmetry elements (plane) but may have rotational axis are said to be as a chiral molecule. The staggered
(b)
Interpretation:
The symmetry elements and point group of chair and boat cyclohexane have to be stated. Whether the molecule is polar or not has to be stated. Whether the molecule is chiral or not has to be stated
Concept introduction:
As mentioned in the concept introduction in part (a).
(b)
Answer to Problem 10A.1P
The symmetry elements of the chair form of cyclohexane are
The chair form of cyclohexane molecule is nonpolar and achiral.
The symmetry elements of the boat form of cyclohexane are
The boat form of cyclohexane molecule is polar and achiral.
Explanation of Solution
The structure of the chair form of cyclohexane is shown below in Figure 3.
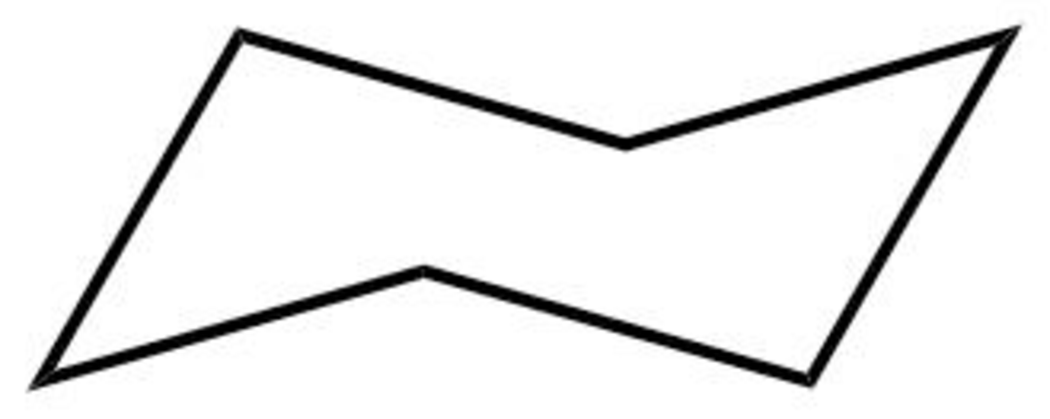
Figure 3
The chair form of cyclohexane has a principal axis
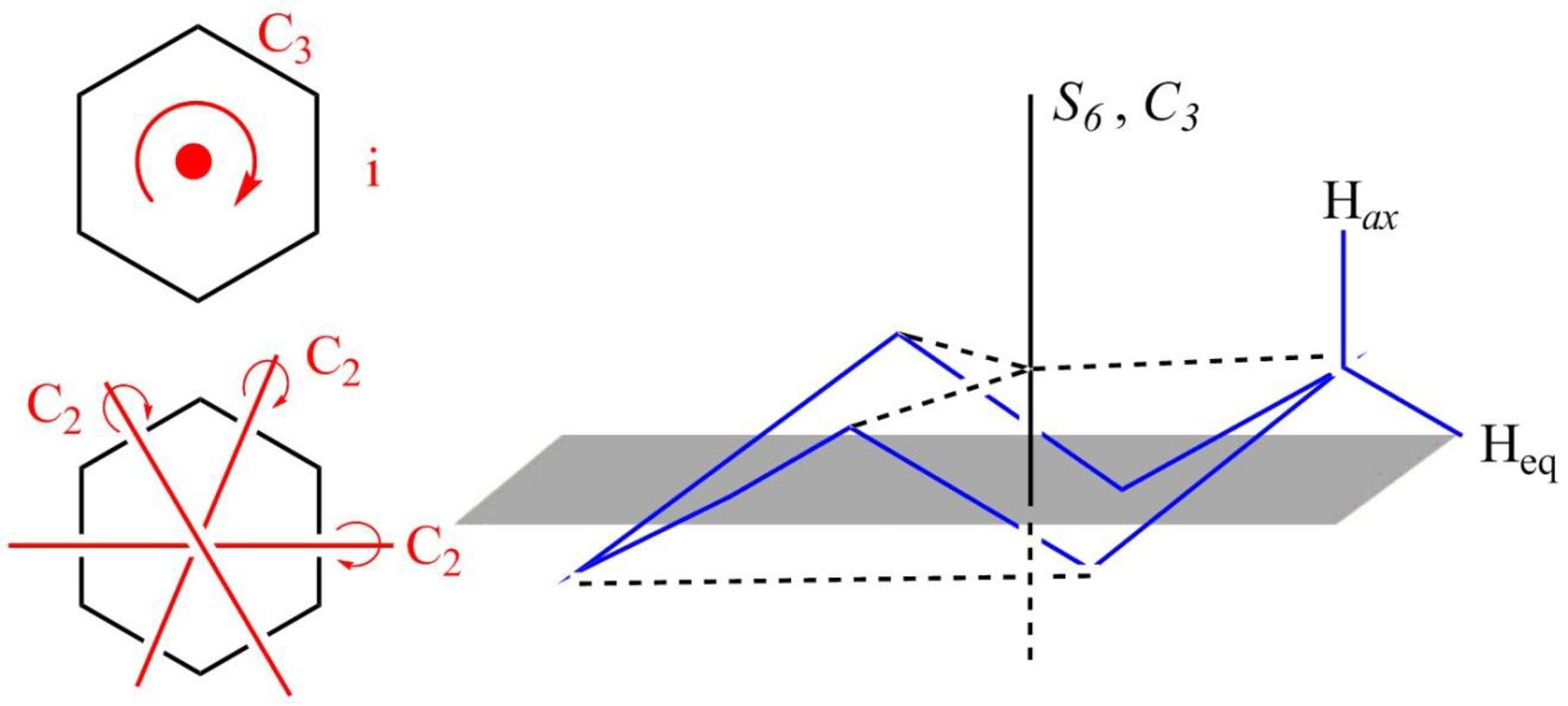
Figure 4
The symmetry elements of the chair form of cyclohexane are
The molecules which have point group
The molecules which do not have any reflective symmetry elements (palne) but may have rotational axis are said to be as a chiral molecule. The chair form of cyclohexane has three
The structure of the boat form of cyclohexane is shown below in Figure 5.
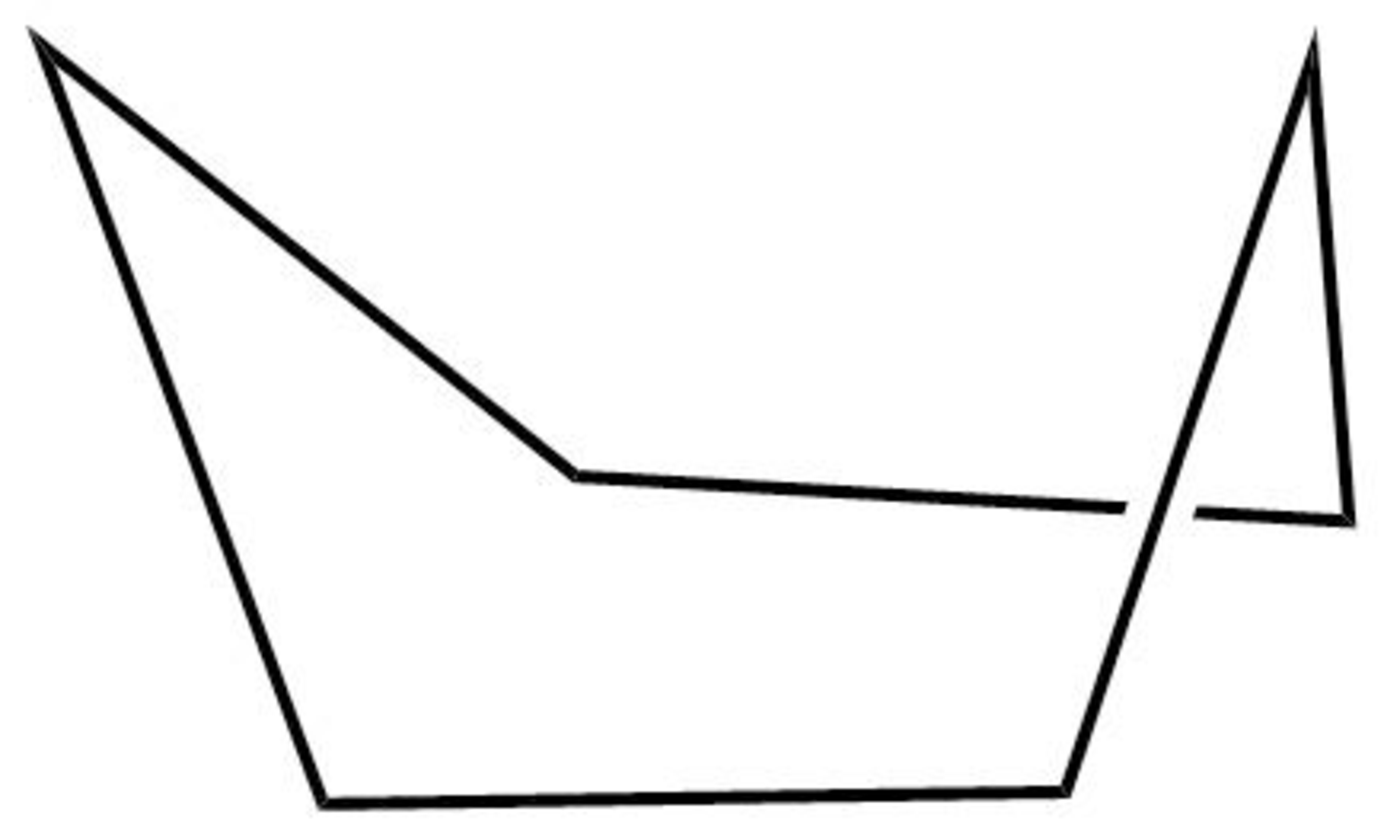
Figure 5
The boat form of cyclohexane has a rotation axis
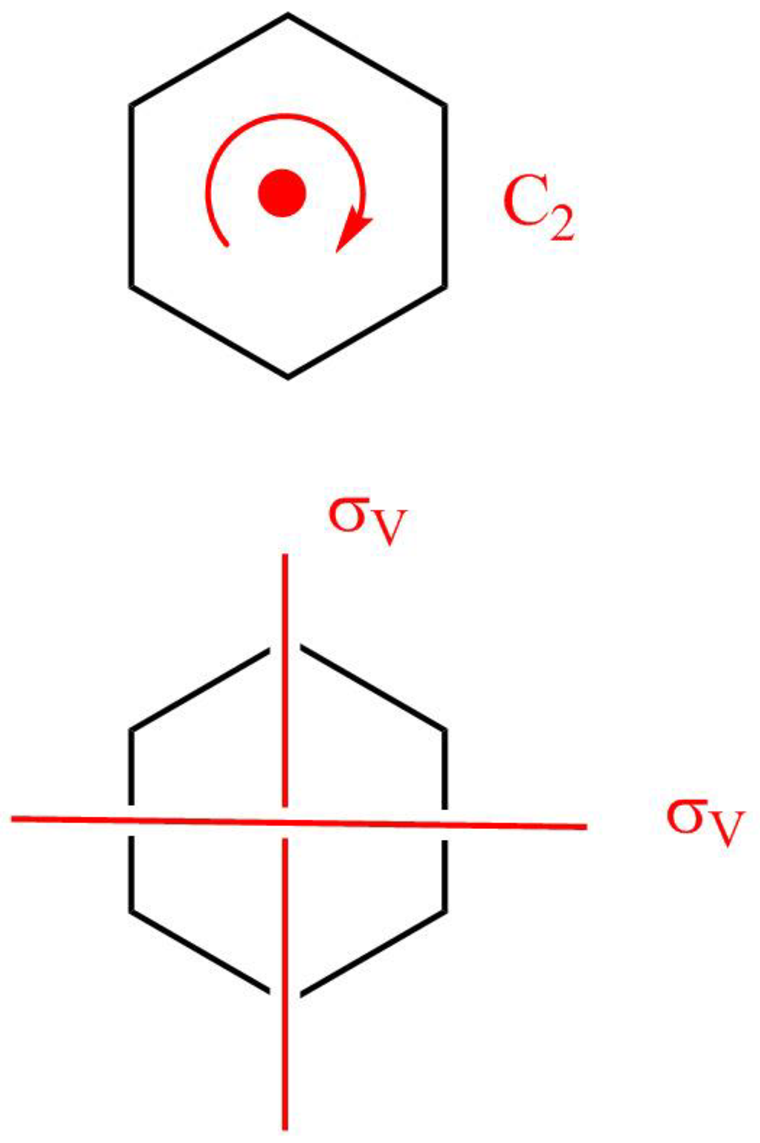
Figure 6
The symmetry elements of the boat form of cyclohexane are
The molecules which have point group
The molecules which do not have any reflective symmetry elements (plane) but may have rotational axis are said to be as a chiral molecule. The boat form of cyclohexane has two
(c)
Interpretation:
The symmetry elements and point group of diborane
Concept introduction:
As mentioned in the concept introduction in part (a).
(c)
Answer to Problem 10A.1P
The symmetry elements of diborane
The diborane
Explanation of Solution
The structure of diborane is shown below in Figure 7.
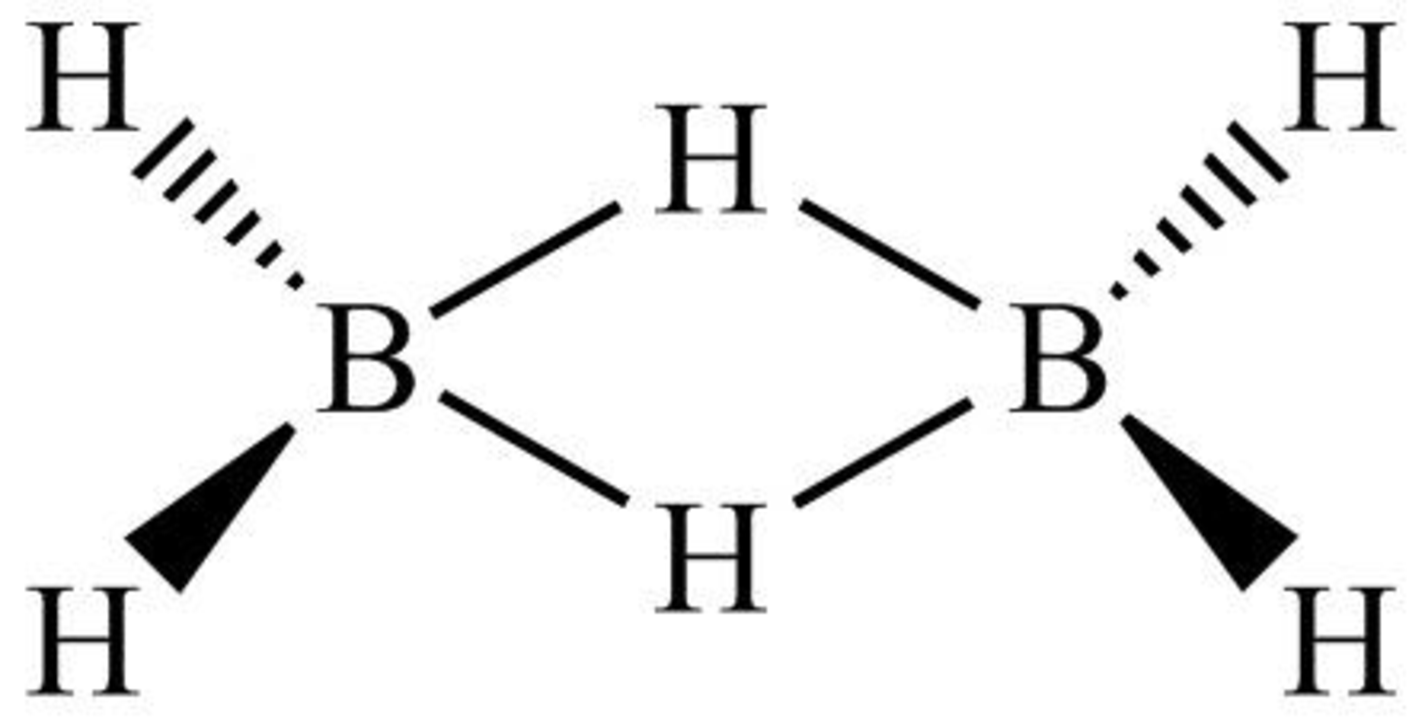
Figure 7
Diborane
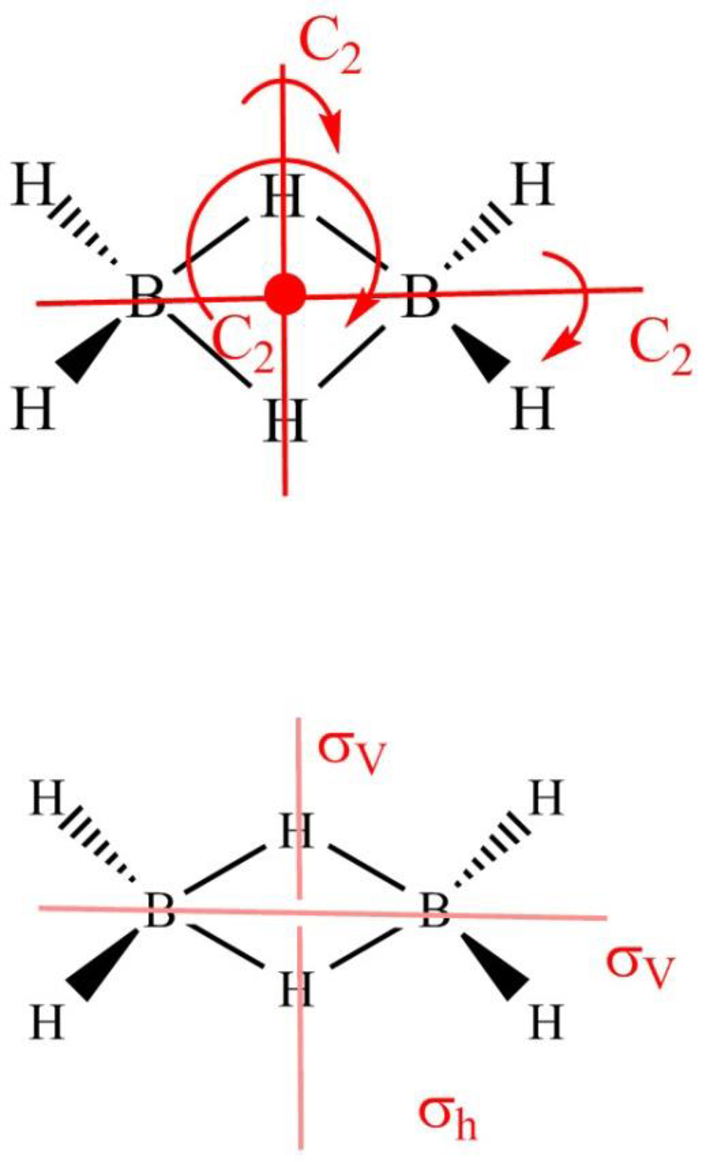
Figure 8
The symmetry elements of diborane
The molecules which have point group
The molecules which do not have any reflective symmetry elements (plane) but may have rotational axis are said to be as a chiral molecule. The diborane
(d)
Interpretation:
The symmetry elements and point group of
Concept introduction:
As mentioned in the concept introduction in part (a).
(d)
Answer to Problem 10A.1P
The symmetry elements of
The
Explanation of Solution
The structure of
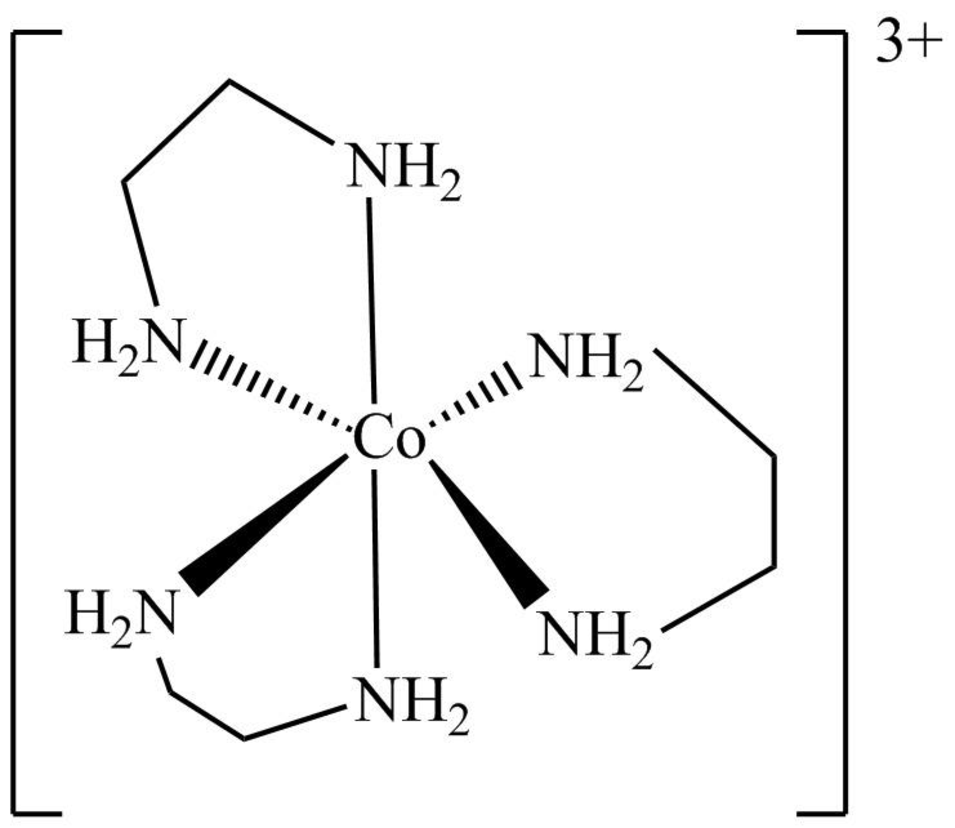
Figure 9
The
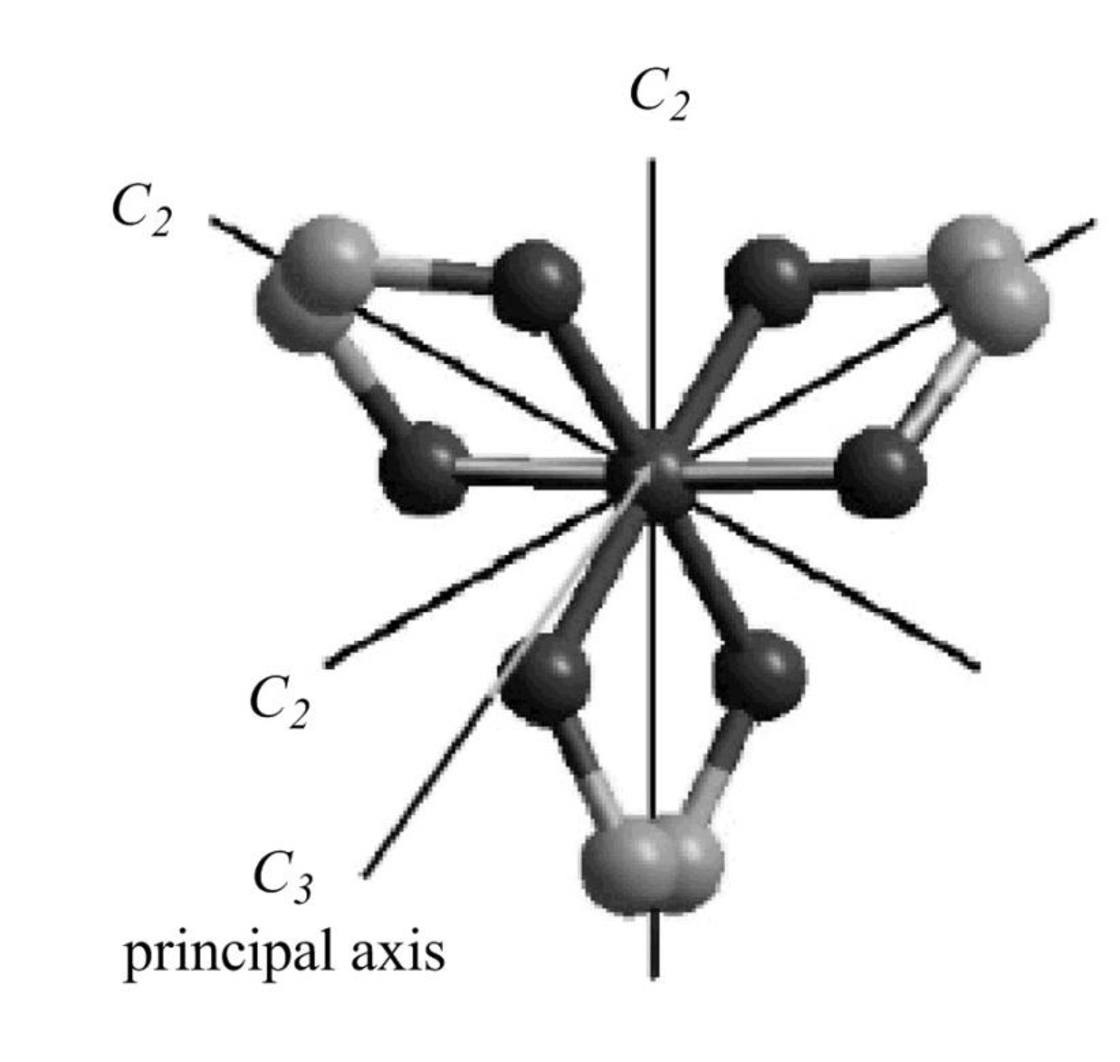
Figure 10
The symmetry elements of
The molecules which have point group
The molecules which do not have any reflective symmetry elements (plane) but may have rotational axis are said to be as a chiral molecule. The
(e)
Interpretation:
The symmetry elements and point group of crown-shaped
Concept introduction:
As mentioned in the concept introduction in part (a).
(e)
Answer to Problem 10A.1P
The symmetry elements of crown-shaped
The crown-shaped
Explanation of Solution
The structure of the crown-shaped
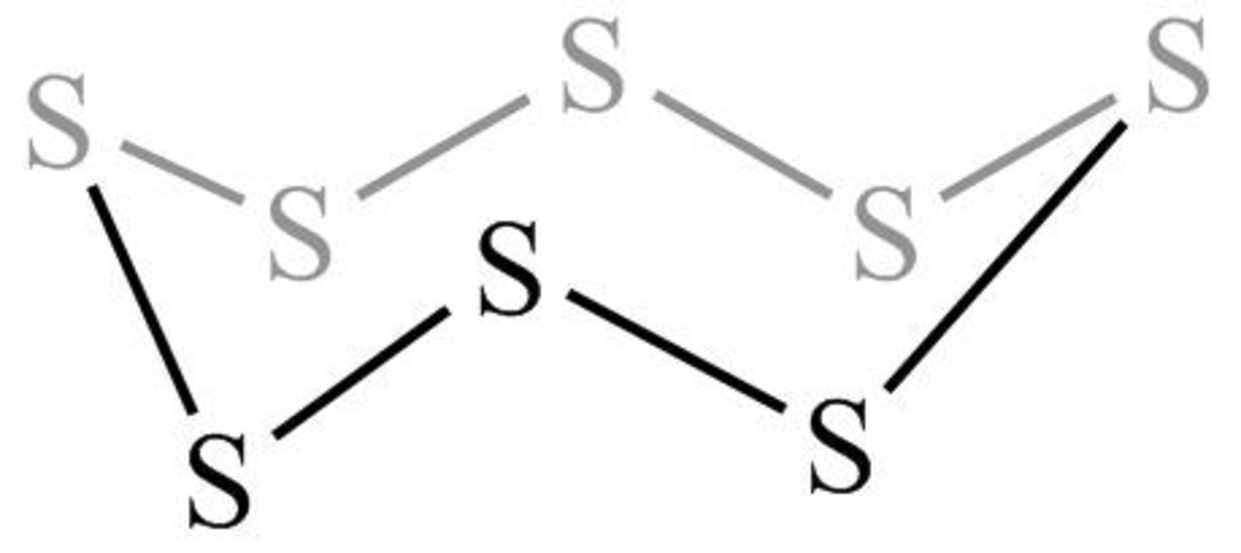
Figure 11
The crown-shaped
The symmetry elements of crown-shaped
The molecules which have point group
The molecules which do not have any reflective symmetry elements (plane) but may have rotational axis are said to be as a chiral molecule. The crown-shaped
Want to see more full solutions like this?
Chapter 10 Solutions
PHYSICAL CHEMISTRY. VOL.1+2 (LL)(11TH)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





