
Concept explainers
- 12. The ideal gas law assumes that molecules bounce around and have negligible volume themselves. This is not always true. To compensate for the simplifying assumptions of the ideal gas law, the Dutch scientist Johannes van der Waals developed a “real” gas law that uses several factors to account for molecular volume and intermolecular attraction. He was awarded the Nobel Prize in 1910 for his work. The van der Waals equation is as follows:
 P, V, n, R, and T are the same quantities as found in the ideal gas law. The constant a is a correction for intermolecular forces [atm L2/mol2], and the constant b accounts for molecular volume [L/mol]. Each of these factors must be determined by experiment.
P, V, n, R, and T are the same quantities as found in the ideal gas law. The constant a is a correction for intermolecular forces [atm L2/mol2], and the constant b accounts for molecular volume [L/mol]. Each of these factors must be determined by experiment.
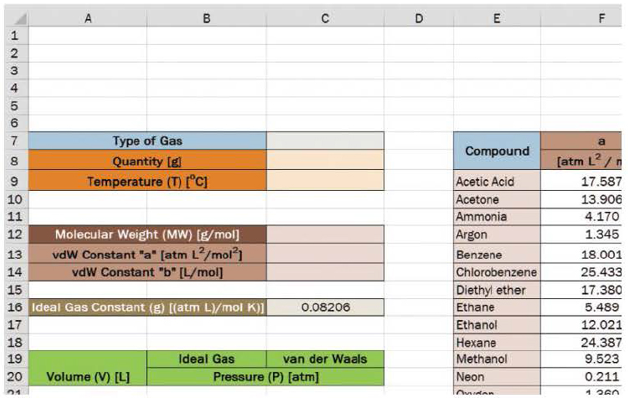
Create a worksheet using the provided template. The molecular weight, a, and b should automatically fill in after the user selects the type of gas in cell B7. The user will also set the quantity of gas and the temperature of the system.
Next, create a column of volume beginning in A21 at 0.5 liters and increasing in increments of 0.1 liters to a volume of 5 liters.
In column B, calculate the pressure (P, in atmospheres [atm]) using the ideal gas law.
In column C, calculate the pressure (P, in atmospheres [atm]) using the van der Waals equation.
Hint
Use data validation and lookup expressions using the data found in the table located in E7 to H26 in the workbook provided.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Thinking Like an Engineer: An Active Approach, Student Value Edition Plus MyLab Engineering with Pearson eText - Access Card Package (4th Edition)
Additional Engineering Textbook Solutions
Machine Tool Practices (10th Edition)
Automotive Technology: Principles, Diagnosis, and Service (5th Edition)
Heat and Mass Transfer: Fundamentals and Applications
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
HEAT+MASS TRANSFER:FUND.+APPL.
Vector Mechanics for Engineers: Statics and Dynamics
- Entropy is one of the important coordinates in thermodynamics. Entropy shows the ratio of heat absorbed or rejected by temperature so that it can indicate the direction of heat flow. Entropy is useful for knowing the internal energy efficiency. Derive an equation for the change in the internal energy of a gas that satisfies the van der Waals equation of state. Assume the value of Cv satisfies the relationship: Cv=c1 + c2T, with c1 and c2 constant *application of the 3rd law of thermodynamicsarrow_forwardQuestion 6. The volume of the gas in a closed container with an initial temperature of 20°C is 7 m³ and the pressure is 6 bar. When the volume is brought to 3 m³, the temperature in the container becomes 35° C, what is the pressure of the gas.arrow_forward1. When the pressure of the evaporator chamber is decreased, the boiling point of the solutions inside the chamber will: 2. It states that for a given concentration, the boiling point of the solution is a linear function of the boiling point of the solvent. a.Boiling point rise b.Enthalpy-concentration chart c.Duhring's rule d.Dalton's law 3.arrow_forward
- 49.) When control volume is losing heat, the rate of heat transfer is negative (sign convention). Select one: True False 47.) The properties of the saturated liquid are the same whether it exists alone or in a mixture with saturated vapor. Select one: True Falsearrow_forwardScenario 14.1 — Vidalia OnionsWhen Vidalia onions are ready, the entire 13 county (plus parts of seven other counties) area in Georgia where they are grown is devoted to the harvest, packaging and shipping of this delicacy. The Onion Shack currently purchases 120,000 bags of onions each year at a price of $100 per bag (a bag is ten pounds). Onion Shack has historically purchased in lots of 3000 bags. Transit time and capacity vary as indicated in the table (lead time is 2 days more than transit time). The safety inventory that Onion Shack requires is 30% of lead time demand and the holding cost for a bag is 15% of cost. Carrier Quantity Shipped (cwt) Quantity shipped (bags) Shipping cost ($/cwt) Transit time (days) Mashburn Rail 200+ 2000+ $2.10 5 Sylvia Express 100+ 1000+ $2.35 3 Anita Enterprises 250+ 2500+ $1.35 3 Smith Trucking 250+ 2500+ $1.00 3 Use Scenario 14.1 to determine the inventory holding cost of the Anita Enterprises option if the Onion Shack purchases…arrow_forwardA coal sample gave the following analysis by weight, Carbon 66.9 per cent, Hydrogen 6.58 per cent, Oxygen 8.77 per cent, the remainder being incombustible. For 20% excess air , determine actual weight of air required per kg of coal. Round your answer to two decimal places.arrow_forward
- The composition of a liquid with suspended solid particles is generally characterized by the fraction of solid particles either by weight or mass, Cs, mass = ms/mm or by volume, Cs, vol = Vs /Vm where m is mass and V is volume. The subscripts s and m indicate solid and mixture, respectively. Develop an expression for the specific gravity of a waterbased suspension in terms of Cs, mass and Cs, vol.arrow_forwardWhich of the following statements best describe for a typical diatomic molecule of an ideal gas eg. N2 at room temperature (300k)a. the spacing between translational levels is less than KbTb. the spacing between rotational levels is less than KbTc. the spacing between vocational levels is less than KbTd. the spacing between the ground and first excited electronic states is less than kbT.e. all of the abovef. none of the aboveg. the first and third answerh. the first and second answerarrow_forward11. 100 g of water are mixed with 150 g of alcohol (). What is the specific gravity of the resulting mixtures, assuming the fluids mixed completely.arrow_forward
- The ideal gas law relates the pressure P, volume V, absolute temperature T (Kelvin), and amount of gas n. The law is P = mAT where R is the gas constant. An engineer must design a large natural gas storage tank to be expandable to maintain the pressure constant at 2.2 atmospheres. In December when the temperature is -15°C, the volume of gas in the tank is 28,500 ft°. What will the volume of the same quantity of gas be in July when the temperature is 31°C? (Hint: Use the fact that n, R, and P are constant in this problem. Note also that Kelvin = °C+273.2)arrow_forwardSteam at 100 bar, 3200 C expands isothermally in a cylinder behind a piston to a pressure of 10 bar. Solve for the work done per kilogram of steam.arrow_forwardThe specific gravity of natural gas is A. 0.08. B. 1.00. C. 0.42. D. 0.60.arrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
