
Concept explainers
(a)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and n-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
In chemistry, structure is the arrangement of
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
(a)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
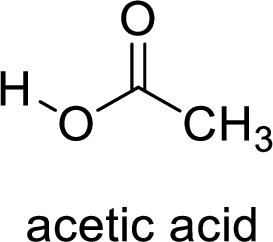
Alcohol is,
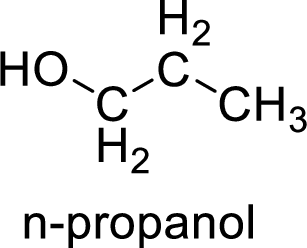
Here, the
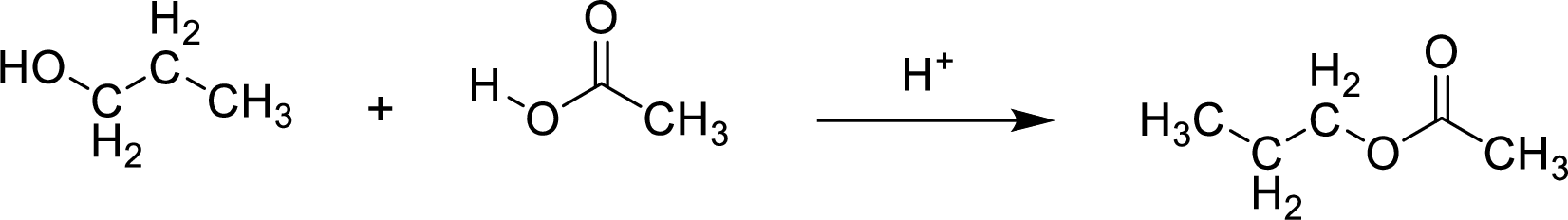
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
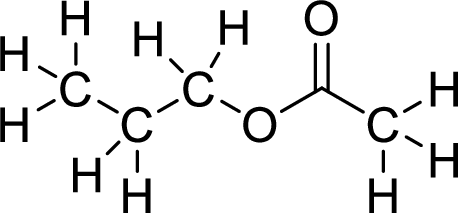
Therefore,
The structural formulas for the ester formed is,
(b)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and iso-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
(b)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
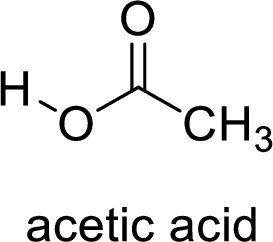
Alcohol is,
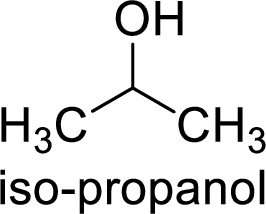
Here, the
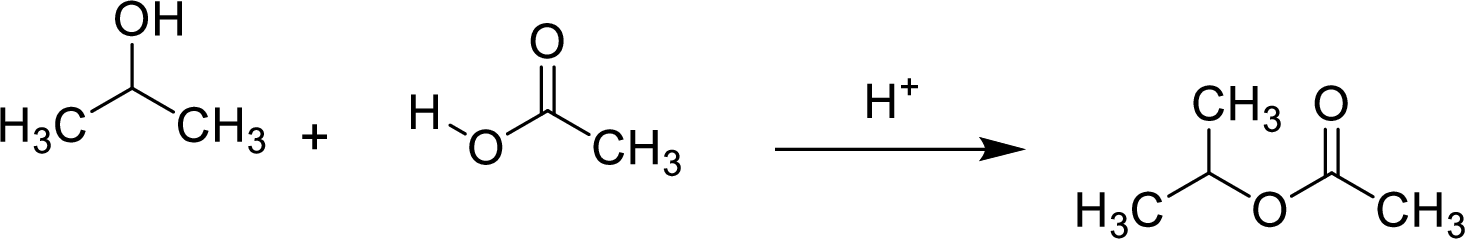
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
Therefore,
The structural formulas for the ester formed is,
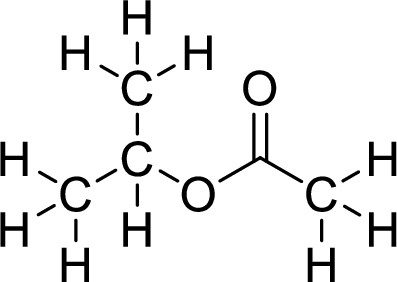
(c)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and iso-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds
- ✓ H atoms are shown
(c)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
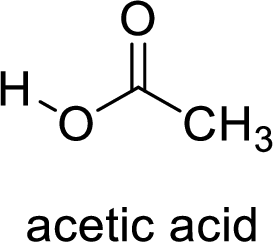
Alcohol is,
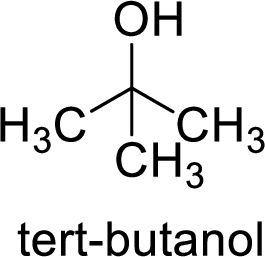
Here, the
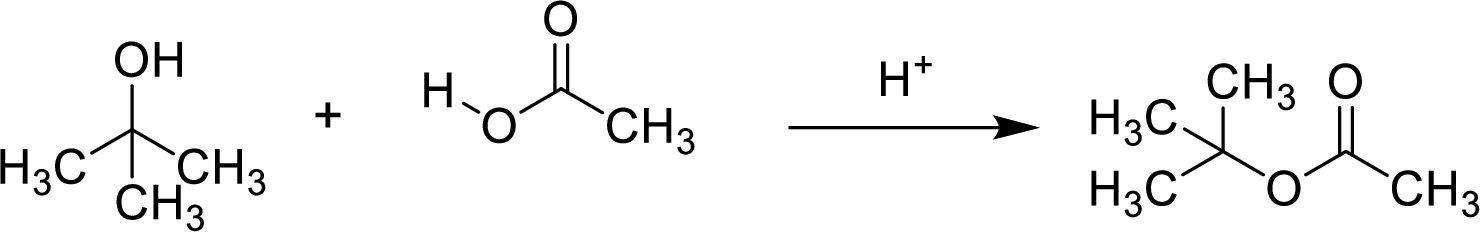
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
Therefore,
The structural formulas for the ester formed is,
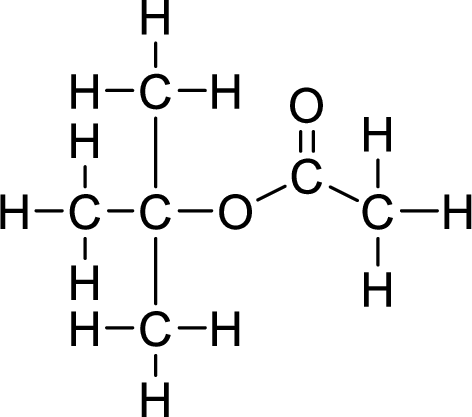
Want to see more full solutions like this?
Chapter 10 Solutions
Laboratory Manual Chemistry in Context
- Alcohols are very useful starting materials for the production of many different compounds. The following conversions, starting with 1-butanol, can be carried out in two or more steps. Show the steps (reactants/catalysts) you would follow to carry out the conversions, drawing the formula for the organic product in each step. For each step, a major product must be produced. (See Exercise 62.) (Hint: In the presence of H+, an alcohol is converted into an alkene and water. This is the exact reverse of the reaction of adding water to an alkene to form an alcohol.) a. 1-butanol butane b. 1-butanol 2-butanonearrow_forwardIn an esterification reaction, a carboxylic acid reacts with an excess of alcohol in acidic conditions to form an ester. Draw the structure of the ester product in the reaction between pentanoic acid and 1‑propanol.arrow_forwardWrite structures of different isomers formed by C6 H10 . Also write IUPAC names of the all the isomersarrow_forward
- What elements compose: a) the hydroxyl group b) the carbonyl group c) an aldehyde group d) a ketone grouparrow_forward(a) When the metallic element sodium combines with the nonmetallic element bromine, Br2(l), how can you determine the chemical formula of the product? How do you know whether the product is a solid, liquid, or gas at room temperature? Write the balanced chemical equation for the reaction. (b) When a hydrocarbon burns in air, what reactant besides the hydrocarbon is involved in the reaction? What products are formed? Write a balanced chemical equation for the combustion of benzene C6H6(l), in air.arrow_forward1. What is formed when a halogen molecule replaces hydrogen molecule(s) in an aromatic hydrocarbon? 2. What is formed when hydrogen molecules are replaced in an aliphatic hydrocarbon by halogen molecules? 3. What are a class of chemical compounds containing a hydroxyl group (-OH) bonded directly to an aromatic hydrocarbon group (like benzene)? 4. used to test for the presence of aromatic compounds in alcohols? 5. The dehydration of alcohols in the formation of ethers happens at _________.arrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





