
Concept explainers
(a)
Interpretation:
Pentane has higher melting point than decane has to be indicated as true or false.
(a)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. Decane contains ten carbon atoms and twenty two hydrogen atoms. As the molar mass increases, the London Dispersion forces become stronger and as a result melting and boiling points will also increase. Therefore, the given statement is false.
(b)
Interpretation:
Pentane is a substituted hydrocarbon has to be indicated as true or false.
(b)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

As seen from the above structure, there are no substituents present on the carbon chain. Hence, the given statement is a false one.
(c)
Interpretation:
Pentane is a saturated hydrocarbon has to be indicated as true or false.
(c)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

As there is only single bonds present between the carbon atoms in the entire molecule as shown above, pentane is a saturated hydrocarbon only. The given statement is true.
(d)
Interpretation:
Pentane is nonpolar has to be indicated as true or false.
(d)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

Pentane contains only carbon and hydrogen atom. Therefore, this is a nonpolar molecule only. Given statement is true.
(e)
Interpretation:
Pentane has structural formula of
(e)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. It is a linear
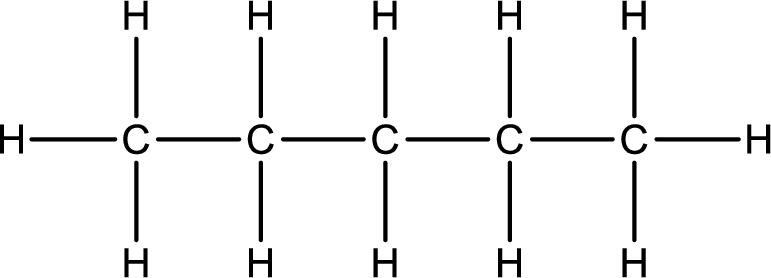
Molecular formula of pentane is only
(f)
Interpretation:
Pentane has weak London Dispersion forces than octane has to be indicated as true or false.
(f)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. Octane contains eight carbon atoms and eighteen hydrogen atoms. As the molar mass increases, the London Dispersion forces become stronger. This means pentane will have weaker London dispersion forces than octane. Hence, the given statement is true.
(g)
Interpretation:
Pentane is a gas at room temperature has to be indicated as true or false.
(g)
Explanation of Solution
Alkanes with one to four carbon atoms are gases at room temperature, and alkanes with five to seventeen carbon atoms are colorless liquids at room temperature. Pentane contains five carbon atoms in its skeleton. Hence, pentane is a liquid at room temperature. Therefore, the given statement is false.
(h)
Interpretation:
Pentane has only carbon‑carbon single bonds and carbon‑hydrogen single bonds has to be indicated as true or false.
(h)
Explanation of Solution
Pentane contains five carbon atoms and twelve hydrogen atoms. The structure of pentane can be given as,

As there are no multiple bonds present in the above structure, pentane contains only single bonds between carbon and carbon, and carbon and hydrogen. Therefore, the given statement is true.
(i)
Interpretation:
Per mol of Pentane requires
(i)
Explanation of Solution
Pentane has a molecular formula as
Balanced chemical equation for the complete combustion of pentane can be given as,
As from the above equation it is understood that one mol of pentane requires eight mol of oxygen for complete combustion, the given statement is true.
(j)
Interpretation:
Pentane is a structural isomer of 2-methylpropane has to be indicated as true or false.
(j)
Explanation of Solution
Pentane has a molecular formula as
(k)
Interpretation:
Pentane on reaction with
(k)
Explanation of Solution
Pentane is a saturated hydrocarbon. It undergoes only substitution reaction and not addition reactions. Pentane does not undergo reaction with hydrogen bromide to form 1-bromopropane, 2-bromopropane, and 3-bromopropane. Therefore, the given statement is false.
Want to see more full solutions like this?
Chapter 10 Solutions
GEN ORGANIC CHM LL W/CONNECT
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





