
Concept explainers
If one were to try to draw the simplest Lewis structure fur molecular oxygen, the result might be the following 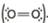
However, it is known from the properties of molecular oxygen and experiments that
(a) What type of orbital is represented by HOMO-3 and HOMO-2? (Hint: What types of orbitals are possible for second-row elements like oxygen, and which orbitals have already been used?)
(b) What type of orbital is HOMO-1? [Hint: The
(c) The orbitals designated HOMO and LUMO in
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Additional Science Textbook Solutions
Chemistry: Matter and Change
Chemistry: A Molecular Approach
General Chemistry: Atoms First
Introductory Chemistry (6th Edition)
Fundamentals of Heat and Mass Transfer
Principles of Chemistry: A Molecular Approach (3rd Edition)
- Aspirin, or acetylsalicylic acid, has the formula C9H8O4 and the skeleton structure (a) Complete the Lewis structure and give the number of bonds and bonds in aspirin. (b) What is the hybridization about the CO2H carbon atom (colored blue)? (c) What is the hybridization about the carbon atom in the benzene-like ring that is bonded to an oxygen atom (colored red)? Also, what is the hybridization of the oxygen atom bonded to this carbon atom?arrow_forwardConsider the polyatomic ion IO65-. How many pairs of electrons are around the central iodine atom? What is its hybridization? Describe the geometry of the ion.arrow_forwardThe structure of amphetamine, a stimulant, is shown below. (Replacing one H atom on the NH2, or amino, group with CH3 gives methamphetamine a particularly dangerous drug commonly known as speed.) (a) What are the hybrid orbitals used by the C atoms of the C6 ring. by the C atoms of the side chain, and by the N atom? (b) Give approximate values for the bond angles A, B, and C. (c) How many bonds and bonds are in the molerule? (d) Is the molecule polar or nonpolar? (e) Amphetamine reacts readily with a proton (H+) in aqueous solution. Where does this proton attach to the molecule? Explain how the electrostatic potential map predicts this site of protonation.arrow_forward
- Do lone pairs about a central atom affect the hybridization of the central atom? If so, how?arrow_forwardWhy is the concept of hybridization required in valence bond theory?arrow_forwardWhy do we hybtidize atomic orbitals to explain the bonding in covalent compounds? What type of bonds form from hybrid orbitals: sigma or pi? Explain.arrow_forward
- Specify the electron-pair and molecular geometry for each underlined atom in the following list. Describe the hybrid orbital set used by this atom in each molecule or ion. (a) CSe2 (b) SO2 (c) CH2O (d) NH4ssarrow_forwardIn the molecular orbital mode l, compare and contrast bonds with bonds. What orbitals form the bonds and what orbitals form the bonds? Assume the z-axis is the internuclear axis.arrow_forwardSpecify the electron-pair and molecular geometry for each underlined atom in the following list. Describe the hybrid orbital set used by this atom in each molecule or ion. (a) BBr3 (b) CO2 (c) CH2Cl2 (d) CO32arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





