
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 45P
Following is a structural formula for naphthalene. It was first obtained by heating coal to a high temperature in the absence of air (oxygen). At one time, it was used in "mothballs.”
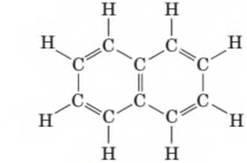
- Predict the shape of naphthalene.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Which of the following statements could be true regarding polar molecules?
Which of the following are polar molecules?
I - H2S II - SF6 III - SF4
Is P2H4 polar or nonpolar? please explain in detail
Chapter 10 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 10.3 - Prob. 10.1QCCh. 10.4 - Prob. 10.2QCCh. 10.4 - Prob. 10.3QCCh. 10.4 - Prob. 10.4QCCh. 10.4 - Prob. 10.5QCCh. 10.4 - Prob. 10.6QCCh. 10 - Prob. 1PCh. 10 - Prob. 2PCh. 10 - 10-9 Is there any difference between vanillin made...Ch. 10 - Prob. 4P
Ch. 10 - 10-11 What important experiment did Wohler carry...Ch. 10 - Prob. 6PCh. 10 - Prob. 7PCh. 10 - Prob. 8PCh. 10 - 10-15 How many electrons are in the valence shell...Ch. 10 - 10-16 What is the relationship between the number...Ch. 10 - Prob. 11PCh. 10 - Prob. 12PCh. 10 - 10-19 Write Lewis structures for these ions. (a)...Ch. 10 - 10-20 Why are the following molecular formulas...Ch. 10 - 10-21 Explain how to use the valence-shell...Ch. 10 - 10-22 Suppose you forget to take into account the...Ch. 10 - Suppose you forget to take into account the...Ch. 10 - Prob. 18PCh. 10 - Prob. 19PCh. 10 - Prob. 20PCh. 10 - 10-27 What is meant by the term functional group?Ch. 10 - 10-28 List three reasons why functional groups are...Ch. 10 - Prob. 23PCh. 10 - Prob. 24PCh. 10 - Prob. 25PCh. 10 - 10-32 Draw a structural formula for the one...Ch. 10 - 10-33 What is the meaning of the term tertiary (...Ch. 10 - Prob. 28PCh. 10 - Draw structural formulas for each of the...Ch. 10 - 10-36 Draw structural formulas for the six ketones...Ch. 10 - 10-37 Draw structural formulas for the eight...Ch. 10 - Prob. 32PCh. 10 - 10-39 (Chemical Connections 10A) How was Taxol...Ch. 10 - Prob. 34PCh. 10 - Prob. 35PCh. 10 - Silicon is immediately below carbon in Group 4A of...Ch. 10 - 10-43 Phosphorus is immediately below nitrogen in...Ch. 10 - Draw the structure for a compound with the...Ch. 10 - 10-45 Draw structural formulas for the eight...Ch. 10 - Prob. 40PCh. 10 - 10-47 Which of these covalent bonds are polar, and...Ch. 10 - Of the bonds in Problem 10-47, which is the most...Ch. 10 - Prob. 43PCh. 10 - Prob. 44PCh. 10 - Following is a structural formula for naphthalene....Ch. 10 - Prob. 46PCh. 10 - Prob. 47PCh. 10 - Urea, (NH.,)2CO, is used in plastics and in fertil...Ch. 10 - Prob. 49PCh. 10 - Prob. 50PCh. 10 - Aspirin is prepared by the reaction of salicylic-...Ch. 10 - Following is the structural formula of acetamide....Ch. 10 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY