
The mole fraction of each component in polycrylonitrile material.
The mole fraction of each component in polystrene material.
The mole fraction of each component in polybutadlene material.
Answer to Problem 72AAP
The mole fraction of each component in polycrylonitrile material is
The mole fraction of each component in polystrene material is
The mole fraction of each component in polybutadlene material is
Explanation of Solution
Show the bonding structure of the polyacryonitrile, polybutadlene, and polystyrene as:
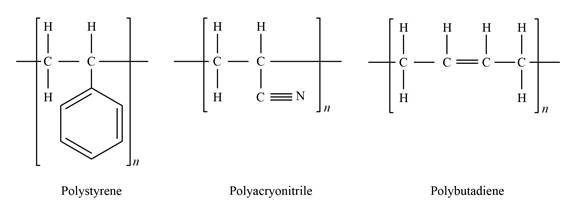
Express the mole fraction of polyacrylonitrile.
Here the number of moles of polystyrene is
Express the mole fraction of polybutadlene.
Express the mole fraction of polystyrene.
Conclusion:
The average molecular mass of the polyacrylonitrile is
The average molecular mass of the polybutadlene is
The average molecular mass of the polystyrene is
Calculate the number of moles of polyacrylonitrile of 100 g of copolymer.
Calculate the number of moles of polybutadlene of 100 g of copolymer.
Calculate the number of moles of polystyrene of 100 g of copolymer.
Substitute
Thus, the mole fraction of each component in polycrylonitrile material is
Substitute
Thus, the mole fraction of each component in polybutalene material is
Substitute
Thus, the mole fraction of each component in polystrene material is
Want to see more full solutions like this?
Chapter 10 Solutions
Connect Access Card For Foundations Of Materials Science And Engineering
- Based on the facts that the free-radical polymerization of ethene is spontaneous and that polymer molecules are less disorganized than the starting monomers, decide whether the polymerization reaction is exothermic or endothermic. Explain.arrow_forwardDraw the structure of a molecule with formula C5H9N that contains functional groups characteristic of (a) an alkyne andan amine; (b) two alkenes and an amine; (c) a nitrile.arrow_forwardWhat is ceiling temperature in thermodynamics of polymerization? Explain the correlation of ceiling temperature with polymers. Show the equations to support your answer.arrow_forward
- Calculate the density of a composite made with 35% Kevlar-49 fibers in an epoxy resin (ρ = 1.1 kg/m3, E = 2.5 GPa). For Kevlar-49, Ef = 131 GPa; density = 1440 kg/m3arrow_forwardFor a given polymer, the activation energy for stress relaxation was measured to be 10 kJ/mol. If the stress relaxation time for this polymer at room temperature is 3,600 s, what would be the relaxation time at 100 ◦C?arrow_forwardDraw the structure of the idealized modulus-temperature for thermoplastic and thermosetscurveand mention all the transitions.arrow_forward
- a) : Define Polymerization. What are the different types of Synthetic polymers ? (b) :. Differentiate between Thermoplastics and Thermosets plastics. Explain in detail the uses of these plastics in the world.arrow_forwardUsing the ‘rule of mixtures’ calculate the modulus and strength, in the fiber direction and perpendicular to the fiber direction, of a glass fiber-epoxy composite plate, given that the weight fraction of glass fiber is 40%. The modulus of the glass fiber is 60 GPa, and the modulus of the epoxy is 3 GPa. The density of glass fiber is 2300 kg/m^3 and the density of epoxy is 1300 kg/m^3. Calculate the tensile strength of the composite using rule of mixtures in the fiber direction given that the tensile strength of the glass fiber is 1 GPa and the epoxy is 70 GPa.arrow_forwardBakelite is one of the widely ___________________ polymer. Select one: Thermoset Elastomers Composite Thermoplasticarrow_forward
- When answering the following questions , ensure that you explain in terms of both the microscopic and macroscopic structure of the materials. A) polymer - explain the difference in structure between a thermoplastic and thermoset material and explain how the structure accounts for the key generic properties of those polymers. B) composite material - for a particular composite and a fibre reinforce composite , explain the difference in structure and whst structual features dictate the properties of the material.arrow_forwardEthylene, C2H4, and tetrafluoroethylene, C2F4, are used to make the polymers polyethylene and polytetrafluoroethylene (Teflon), respectively.(a) Draw the Lewis structures for C2H4 and C2F4, and give theideal H-C-H and F-C-F bond angles.(b) The actual H-C-H and F-C-F bond angles are 117.4° and112.4°, respectively. Explain these deviations.arrow_forwardThere are five different regions of viscoelastic behaviour of amorphous polymers. Describe thebehaviour of polymer chains and interactions in each of the different regions.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





