
Consider a steam power plant that operates on a reheat Rankine cycle. Steam enters the high-pressure turbine at 10 MPa and 500°C and the low-pressure turbine at 1 MPa and 500°C. Steam leaves the condenser as a saturated liquid at a pressure of 10 kPa. The isentropic efficiency of the turbine is 80 percent, and that of the pump is 95 percent. Determine the exergy destruction associated with the heat addition process and the expansion process. Assume a source temperature of 1600 K and a sink temperature of 285 K. Also, determine the exergy of the steam at the boiler exit. Take P0 = 100 kPa.
The exergy destruction associated with the heat addition, the expansion process, and the exergy of the steam at the boiler exit.
Answer to Problem 62P
The exergy destruction during the heating process is
The exergy destruction during the expansion process is
The exergy of the steam at the boiler exit is
Explanation of Solution
Draw the
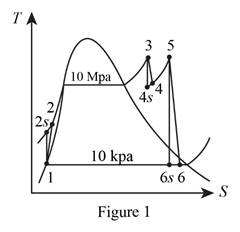
Here, water is the working fluid.
Write the formula for work done by the pump during process 1-2 with the consideration of isentropic efficiency
Here, the specific volume is
Write the formula for enthalpy
Before reheating,
At state
The isentropic efficiency is expressed as follows.
After reheating,
At state 5:
The reheating occurs at constant pressure. Hence, the pressure at state 4 and state 5 are equal.
At state
The steam is expanded to the pressure of
The quality of water at the exit of the L.P turbine (state 6) is expressed as follows (actual).
The enthalpy at state 6 is expressed as follows.
Here, the enthalpy is
The isentropic efficiency is expressed as follows.
Here, the subscript
Write the formula for heat input
Write the formula for the exergy destruction for the combined heat addition and pumping process 1-5.
Here, the entropy
Write the formula for the exergy destruction for pumping process 1-2.
Here, the work input of pump at actual process 1-2 is
Write the formula for exergy destruction for heat addition processes 2-3, 4-5.
Write the formula for the exergy destruction for the expansion process 3-4, 5-6.
Write the formula for exergy of the steam at boiler exit
Here, the enthalpy is
Neglect the kinetic energy
At state 1:
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 3: (H.P. Turbine inlet)
The steam enters the as superheated vapor.
Refer Table A-6, “Superheated water”.
The enthalpy
At state
From Figure 1.
Refer Table A-6, “Superheated water”.
The enthalpy
At state 5: (L.P. Turbine inlet)
The steam is reheated to superheated at the pressure of
Refer Table A-6, “Superheated water”.
The enthalpy
At state
From Figure 1.
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
Here, the sink temperature is equal to the surrounding temperature.
The surrounding pressure
Refer Table A-4, “Saturated water-Temperature table”.
The enthalpy
Conclusion:
Substitute
Substitute
Substitute
Substitute
Substitute
Equation (V).
Substitute
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the vapor enthalpy
The calculated enthalpy at state 6 is greater than the vapor enthalpy at this state. Hence, the steam is at superheated state.
Substitute
Consider the process 1 to 5 (combined heat addition and boiler).
Here,
Substitute 285 K for
Substitute
Substitute
Thus, the exergy destruction during the heating process is
Consider the process 3-4 and 5-6 (H.P. turbine expansion and L.P. turbine expansion).
Here,
Substitute 285 K for
Thus, the exergy destruction during the expansion process is
Substitute
Thus, the exergy of the steam at the boiler exit is
Want to see more full solutions like this?
Chapter 10 Solutions
Thermodynamics - With Connect Access
- Consider a 210-MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500C and is cooled in the condenser at a pressure of 10 kPa. Assuming an isentropic efficiency of 85 percent for both the turbine and the pump, determine the ideal quality (%) of the steam at the turbine exit. (Use 2 decimal places for the final answer.)arrow_forwardConsider a 210-MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500C and is cooled in the condenser at a pressure of 10 kPa. Assuming an isentropic efficiency of 85 percent for both the turbine and the pump, determine the actual quality (%) of the steam at the turbine exit. (Use 2 decimal places for the final answer.)arrow_forwardConsider a 210-MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500C and is cooled in the condenser at a pressure of 10 kPa. Assuming an isentropic efficiency of 85 percent for both the turbine and the pump, determine the actual thermal efficiency (%) of the cycle. (Use 2 decimal places for the final answer.)arrow_forward
- Consider a 210-MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500C and is cooled in the condenser at a pressure of 10 kPa. Assuming an isentropic efficiency of 85 percent for both the turbine and the pump, determine the quality ofthe steam at the turbine exit, the thermal efficiency of the cycle, specific enthalpy (kJ/kg) at the condenser, and actual Qin (kJ/kg).arrow_forwardConsider a steam power plant operating on a simple Rankine cycle. Steam enters the turbine at 15 MPa and 650°C and is condensed in the condenser at a pressure of 15 kPa. Assuming an isentropic efficiency of 84% and 86% for the pump and turbine, respectively, determine the actual thermal efficiency (%). (Use 2 decimal places for the final answer.)arrow_forwardSteam enters the high-pressure turbine of a steam power plant that operates on the reheat Rankine cycle at 10 MPa and 550°C and leaves at 4 MPa. Steam is then reheated to 500°C before it expands to a pressure of 40 kPa. The enthalpy of steam is 3190 kJ/kg at the exit of the high-pressure turbine, and 2550 kJ/kg at the exit of the low-pressure turbine. Disregarding the pump work, the cycle efficiency isarrow_forward
- Consider a steam power plant operating on the ideal Rankine cycle. Steam enters the turbine at 3 MPa and 623 K and is condensed in the condenser at a pressure of 10 kPa. Determine (i) the thermal efficiency of this power plant, (ii) the thermal efficiency if steam is superheated to 873 K instead of 623 K, and (iii) the thermal efficiency if the boiler pressure is raised to 15 MPa while the turbine inlet temperature is maintained at 873 K.arrow_forwardThermodynamics question Consider a steam power plant operating on the simple ideal Rankine cycle. Steam enters the turbine at 3 MPa and 350 °C and is condensed in the condenser at a pressure of 10 kPa. If line number 1 is saturated liquid and assuming 100% isentropic efficiency of the turbine and the pump. Determine: A-work produced in the turbine per unit mass, B-The thermal efficiency of this cycle, C-The heat lost from the condenser, D-maximum possible efficiency of the cycle.arrow_forwardConsider a 210-MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500C and is cooled in the condenser at a pressure of 10 kPa. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the quality of the steam at the turbine exit, (b) the thermal efficiency of the cycle, and (c) the mass flow rate of the steam.arrow_forward
- Thermodynamics 2 Consider a steam power plant that operates on a reheat Rankine cycle and has a net power output of 91 MW. Steam enters the high-pressure turbine at 10 MPa and 500 C and the low-pressure turbine at 1 MPa and 500 C. Steam leaves the condenser as a saturated liquid at a pressure of 10 kPa. The isentropic efficiency of the turbine is 95 percent, and that of the pump is 90 percent. The required power input in the pump in MW.arrow_forwardConsider a steam power plant operating on the ideal Rankine cycle. Steam enters the turbine at 3 MPa and 350°C and is condensed in the condenser at a pressure of 10 kPa. Determine the thermal efficiency of this power plant, the thermal efficiency if steam is superheated to 600°C instead of 350°C, and the thermal efficiency if the boiler pressure is raised to 15 MPa while the turbine inlet temperature is maintained at 600°C. Draw the T-S diagram of each condition.arrow_forwardIn the preliminary design of a power plant, water is chosen as the working fluid and it is determined that the turbine inlet temperature may not exceed 520C. Based on expected cooling water temperatures, the condenser is to operate at a pressure of 0.06 bar. Determine the steam generator pressure required if the isentropic turbine efficiency is 80% and the quality of steam at the turbine exit must be at least 90%.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





