
Concept explainers
The relative rate of radical bromination is 1;82;1640 for 1°;2°;3° hydrogens, respectively. Draw all of the monobrominated products that you might obtain from the radical bromination of the compounds below. Calculate the relative percentage of each.
(a) methylcyclobutane
(b) 3,3-dimethylpentane
(c) 3-methylpentane
a) Methylcyclobutane
Interpretation:
The relative rate of radical bromination is 1:82:1640 for 1°, 2° and 3° hydrogens respectively. The structures of all the monobrominated products obtainable from the radical bromination of methylcyclobutane are to be drawn. The relative percentage of their formation is also to be calculated.
Concept introduction:
During radical bromination all types of hydrogens present in a compound are replaced by bromine to yield different products. The number of each type of hydrogen present in the compound and their relative rates of bromination are calculated seperately. The relative percentage of formation of a particular type of hydrogen can be calculated from the total rate of bromination of all types of hydrogens and that of the particular hydrogen.
To draw:
The structures of all the monobrominated products obtainable from the radical bromination of methylcyclobutane.
To calculate:
The relative percentage of formation of each monobromination product.
Answer to Problem 48AP
Four different monochlorination products are possible by the radical bromination of methylcyclohexane. They are bromomethylcyclobutane(I), 1-bromo-1-methylcyclobutane(II), 1-bromo-2-methylcyclobutane(III) and 1-bromo-3-methylcyclobutane(IV).
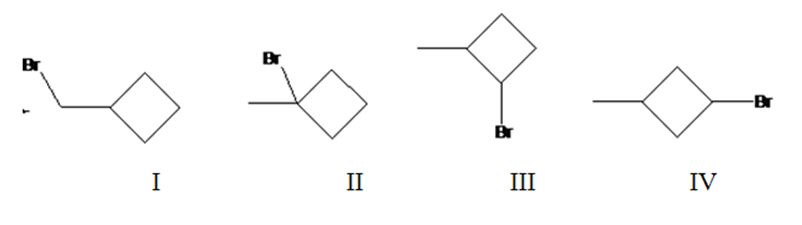
The relative percentage of 1°, 2° and 3° hydrogens in methylcyclobutane is 0.15: 23.1: 76.8.
Explanation of Solution
Methylcyclobutane has four types of hydrogens, one in methyl, a second on C1 to which methyl group is attached, a third one on C2 and C4 and a fourth one on C3. It has three 1° hydrogens, six 2° hydrogens and one 3° hydrogen. Hence four monochlorination products are possible.
The relative rate of radical bromonation of 1° hydrogens = 3 x 1 = 3.
The relative rate of radical bromonation of 2° hydrogens = 6 x 82 = 492.
The relative rate of radical bromonation of 3° hydrogens = 1 x 1640 = 1640.
Total rate of radical bromonation of all hydrogens = 3+492+1640 = 2135.
Therefore the relative percentage of
Radical bromonation of 1° hydrogens = 3/2135 x 100= 0.15%.
Radical bromonation of 2° hydrogens = 492/2135 x 100= 23.1%.
Radical bromonation of 3° hydrogens = 1640/2135 x 100= 76.8%.
Four different monochlorination products are possible by the radical bromination of methylcyclohexane. They are bromomethylcyclobutane(I), 1-bromo-1-methylcyclobutane(II), 1-bromo-2-methylcyclobutane(III) and 1-bromo-3-methylcyclobutane(IV).

The relative percentage of 1°, 2° and 3° hydrogens in methylcyclobutane is 0.15: 23.1: 76.8.
b) 3,3-dimethylpentane
Interpretation:
The relative rate of radical bromination is 1:82:1640 for 1°, 2° and 3° hydrogens respectively. The structures of all the monobrominated products obtainable from the radical bromination of 3,3-dimethylpentane are to be drawn. The relative percentage of their formation is also to be calculated.
Concept introduction:
During radical bromination all types of hydrogens present in a compound are replaced by bromine to yield different products. The number of each type of hydrogen present in the compound and their relative rates of bromination are calculated seperately. The relative percentage of formation of a particular type of hydrogen can be calculated from the total rate of bromination of all types of hydrogens and that of the particular hydrogen.
To draw:
The structures of all the monobrominated products obtainable from the radical bromination of 3,3-dimethylpentane.
To calculate:
The relative percentage of formation of each monobromination product.
Answer to Problem 48AP
Three different monochlorination products are possible by the radical bromination of 3-bromomethyl-3-methylpentane. They are bromomethylcyclobutane(I), 2-bromo-3,3-dimethylpentane(II) and 1-bromo-3,3-dimethylpentane (III).
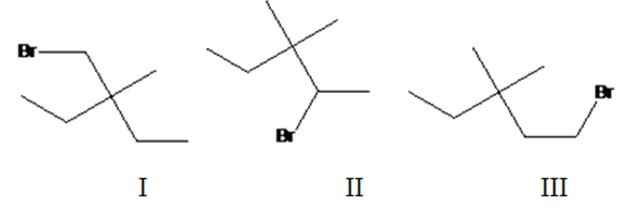
The relative percentage of 10and 20 hydrogens in 3,3-dimethylpentane is 3.6:96.4.
Explanation of Solution
3,3-Dimethylpentane has two types of hydrogens, one in methyl groups and other in CH2 attached to methyl group. Hence two monochlorination products are possible. It has twelve 1° hydrogens and four 2° hydrogen atoms.
The relative rate of radical bromonation of 1° hydrogens = 12 x 1 = 12.
The relative rate of radical bromonation of 2° hydrogens = 4 x 82 = 328.
Total rate of radical bromonation of all hydrogens = 12+328 = 340.
Therefore the relative percentage of
Rdical bromonation of 1° hydrogens = 12/340 x 100= 3.6%.
Rdical bromonation of 2° hydrogens = 328/340 x 100=96.4%.
Three different monochlorination products are possible by the radical bromination of 3-bromomethyl-3-methylpentane. They are bromomethylcyclobutane(I), 2-bromo-3,3-dimethylpentane(II) and 1-bromo-3,3-dimethylpentane (III).

The relative percentage of 1° and 2° hydrogens in 3,3-dimethylpentane is 3.6:96.4.
c) 3-methylpentane
Interpretation:
The relative rate of radical bromination is 1:82:1640 for 1°, 2° and 3° hydrogens respectively. The structures of all the monobrominated products obtainable from the radical bromination of 3-methylpentane are to be drawn. The relative percentage of their formation is also to be calculated.
Concept introduction:
During radical bromination all types of hydrogens present in a compound are replaced by bromine to yield different products. The number of each type of hydrogen present in the compound and their relative rates of bromination are calculated seperately. The relative percentage of formation of a particular type of hydrogen can be calculated from the total rate of bromination of all types of hydrogens and that of the particular hydrogen.
To draw:
The structures of all the monobrominated products obtainable from the radical bromination of 3-methylpentane.
To calculate:
The relative percentage of formation of each monobromination product.
Answer to Problem 48AP
Four different monochlorination products are possible by the radical bromination of 3-methylpentane. They are bromomethylpentane(I), 3-bromo-3-methylpentane(II), 2-bromo-3-methylpentane(III) and 1-bromo-3-methylpentane(IV).
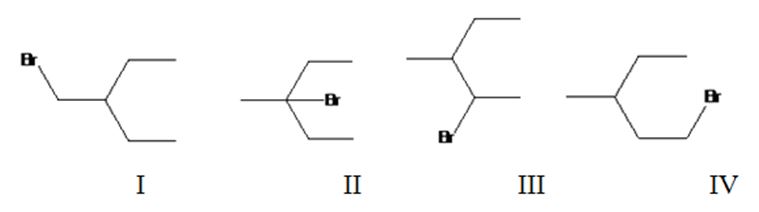
The relative percentage of 1°, 2° and 3° hydrogens in 3,3-dimethylpentane is 0.46:16.6:82.9.
Explanation of Solution
3,3-Dimethylpentane has four types of hydrogens, one in methyl at C1, another methyl groups attached to CH2, a third in C2 and a fourth in CH2. It has nine 1° hydrogens, four 2° hydrogens and one 3° hydrogen. Hence four monochlorination products are possible.
The relative rate of radical bromonation of 1° hydrogens = 9 x 1 = 9.
The relative rate of radical bromonation of 2° hydrogens = 4 x 82 = 328.
The relative rate of radical bromonation of 3° hydrogens = 1 x 1640 = 1640.
Total rate of radical bromonation of all hydrogens = 9+328+1640 = 1977.
Therefore the relative percentage of
Radical bromonation of 1° hydrogens = 9/1977x100= 0.46%.
Radical bromonation of 2° hydrogens = 328/1977x100= 16.6%.
Radical bromonation of 3° hydrogens = 1640/1937x100= 82.9%.
Four different monochlorination products are possible by the radical bromination of 3-methylpentane. They are bromomethylpentane(I), 3-bromo-3-methylpentane(II), 2-bromo-3-methylpentane(III) and 1-bromo-3-methylpentane(IV).

The relative percentage of 1°, 2° and 3° hydrogens in 3,3-dimethylpentane is 0.46:16.6:82.9.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry (Instructor's)
- Which is more stable - a phenyl radical, [C6H5]•, or a benzyl radical, [C6H5CH2]• - and why?arrow_forwardSuppose you were told that each reaction is a substitution reaction, but you were not told the mechanism. Describe how you could conclude from the structure of the haloalkane or cycloalkene, the nucleophile, and the solvent that each reaction is an SN1 reaction.arrow_forwardConsider the substitution reaction that takes place when (R)-3-bromo-3-methylhexane is treated with sodium methoxide. Deduce the mechanism of this reaction working step by step. Write all possible products showing the stereochemistry.arrow_forward
- Bromine reacts with alkenes in methanol according to the equation: - When this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield). Which of the following is the structure more reasonable for this compound?. Explain your reasoning through acorresponding mechanism.arrow_forward(1) Predict the outcome of the addition of HBr to (a) trans-2-pentene, (b) 2-methyl-2-butene, and (c) 4-methylcyclohexene. How many isomers can be formed in each case? (2) Addition of HBr to 3,3-dimethyl-1-butene gives a mixture of two isomeric alkyl bromide products. Draw structures for the two products, and give a mechanistic explanation for their formation.arrow_forwardThe reaction of (S)-2-bromopentane with potassium cyanide to yield 2-methylpentanenitrile (2-cyanopentane) occurs via a nucleophilic substitution pathway. The reaction is 100% stereospecific. Please explain in complete words what this observation tells you about the mechanism of the reaction.arrow_forward
- 1. What is the function of CH2Cl2 in the bromination reactions? Why can it fulfill this role?2. In not more than three (3) sentences, explain why terminal alkynes are acidic.3. What impurities are removed when acetylene gas is made to pass through an acidified solution of CuSO4?4. Explain the difference in the rate of free-radical bromination reactions of toluene and cyclohexane.5. Give the reagent or chemical test that would differentiate the following pairs o fcompounds. Provide only the reagents or chemical tests discussed in the module. Write chemical equations for the reactions involved. a. benzene and ethylbenzeneb. 1-butyne and 2-butynec. 2-methylpentane and 2-methyl-2-pentened. toluene and 1-methylcyclohexenearrow_forwardYou have been tasked with the job of converting cyclohexane to iodocyclohexane. Radical iodination is not a feasible process (it is not thermodynamically favorable), so you cannot directly iodinate the starting cycloalkane that way. Propose an alternative strategy for performing the transformation of cyclohexane to iodocyclohexane. [Hint: you might not be able to do it in one step]arrow_forwardWith reference to the indicated C-H bonds in the following compound: a. Rank the C-H bonds in order of increasing bond strength b. Draw the radical resulting from cleavage of each C-H bond, and classify it as 1º, 2º, or 3º c. Rank the radicals in order of increasing stability d. Rank the C-H bonds in order of increasing ease of H abstraction in a radical halogenation reaction.arrow_forward
- Bromine reacts with alkenes in methanol according to the equation (see image 1). When this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield) a) Which of the following is the structure more reasonable for this compound? (see image 2) b) Explain your reasoning through a corresponding mechanismarrow_forwardOn acid-catalyzed dehydration, 1-butanol (CH3CH2CH2CH2OH) can be converted to 1-butene. Write out an equation for the reaction Assign each the appropriate symbol for the mechanism of the reaction (E1 or E2) Draw a suitable mechanism for the reactionarrow_forwardShow stereo chemistry and possible outcomes and the steps for the reaction.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

