
(a)
Interpretation:
The hybridization of the central atom that corresponds to a trigonal planar arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(a)
Answer to Problem 11.1P
The hybridization of the central atom in trigonal planar is
Explanation of Solution
The molecule that has trigonal planar geometry is
The partial orbital diagram of an isolated
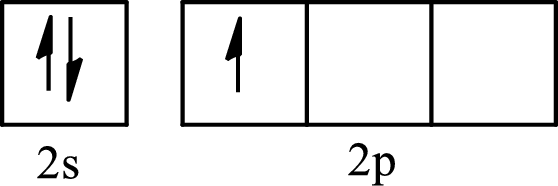
The partial orbital diagram of a hybridized
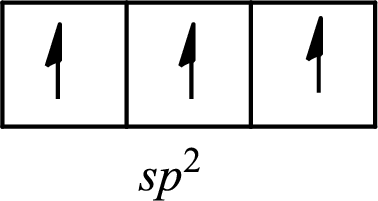
The half-filled
The hybridization of the central atom in trigonal planar is
(b)
Interpretation:
The hybridization of the central atom that corresponds to the octahedral arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(b)
Answer to Problem 11.1P
The hybridization of the central atom in octahedral is
Explanation of Solution
The molecule that has octahedral geometry is
The partial orbital diagram of an isolated
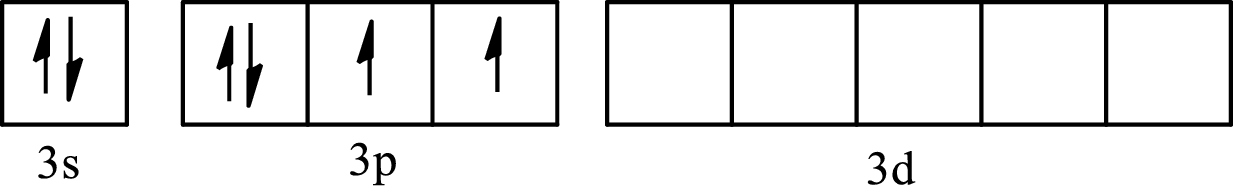
The partial orbital diagram of a hybridized
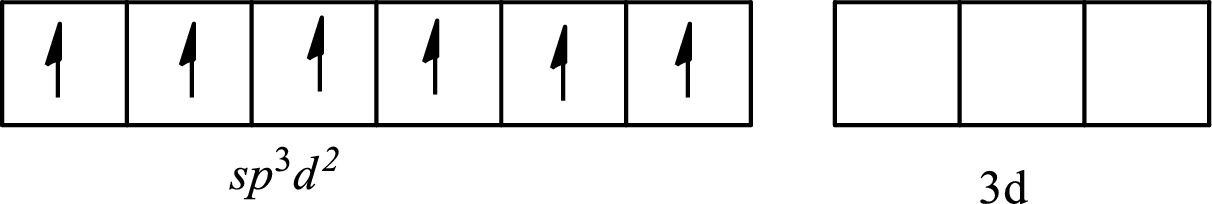
The six half-filled
The hybridization of the central atom in octahedral is
(c)
Interpretation:
The hybridization of the central atom that corresponds to a linear arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(c)
Answer to Problem 11.1P
The hybridization of the central atom in a linear arrangement is
Explanation of Solution
The molecule that has a linear arrangement is
The partial orbital diagram of an isolated
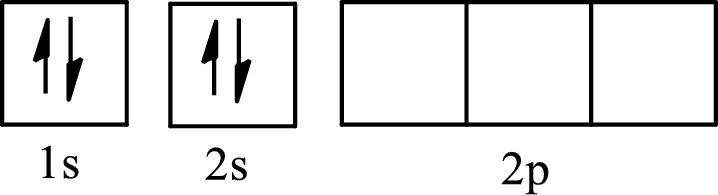
The partial orbital diagram of a hybridized
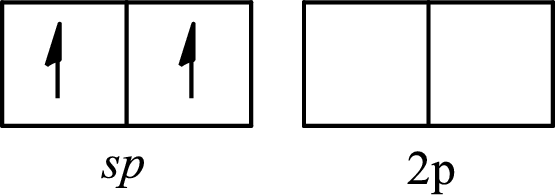
The two half-filled
The hybridization of the central atom in a linear arrangement is
(d)
Interpretation:
The hybridization of the central atom that corresponds to a tetrahedral arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(d)
Answer to Problem 11.1P
The hybridization of the central atom in a tetrahedral arrangement is
Explanation of Solution
The molecule that has a tetrahedral arrangement is
The partial orbital diagram of an isolated
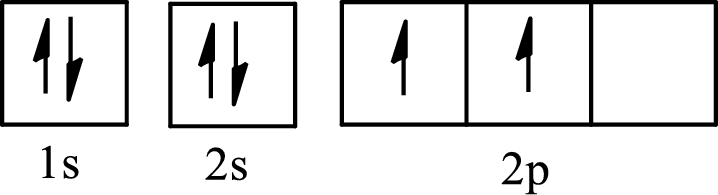
The partial orbital diagram of a hybridized
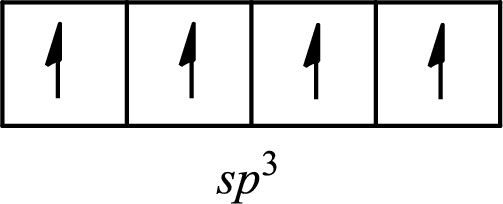
The four half-filled
The hybridization of the central atom in a tetrahedral arrangement is
(e)
Interpretation:
The hybridization of the central atom that corresponds to a trigonal bipyramidal arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(e)
Answer to Problem 11.1P
The hybridization of the central atom in a trigonal bipyramidal arrangement is
Explanation of Solution
The molecule that has a trigonal bipyramidal arrangement is
The partial orbital diagram of an isolated
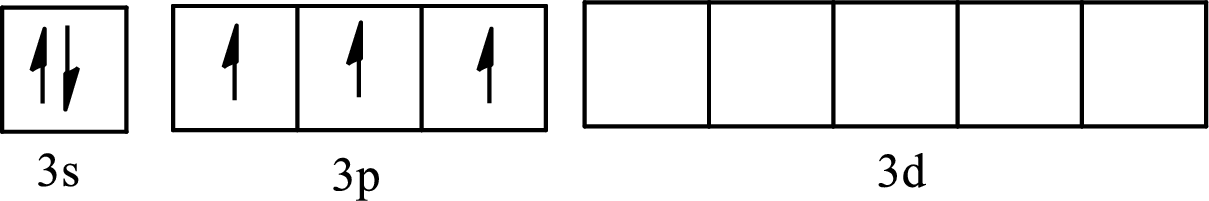
The partial orbital diagram of a hybridized
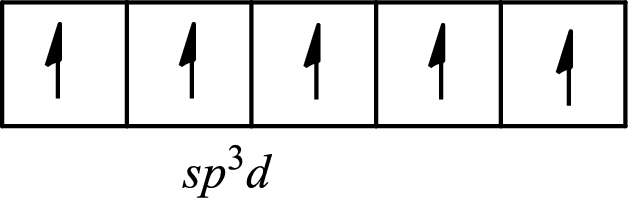
The five half-filled
The hybridization of the central atom in a trigonal bipyramidal arrangement is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





