
Concept explainers
(a)
Interpretation:
The order of decreasing bond energy of
Concept introduction:
A molecular orbital diagram is a tool that is used to describe the
(a)
Answer to Problem 11.37P
The order of decreasing bond energy of
Explanation of Solution
The molecular orbital diagram of
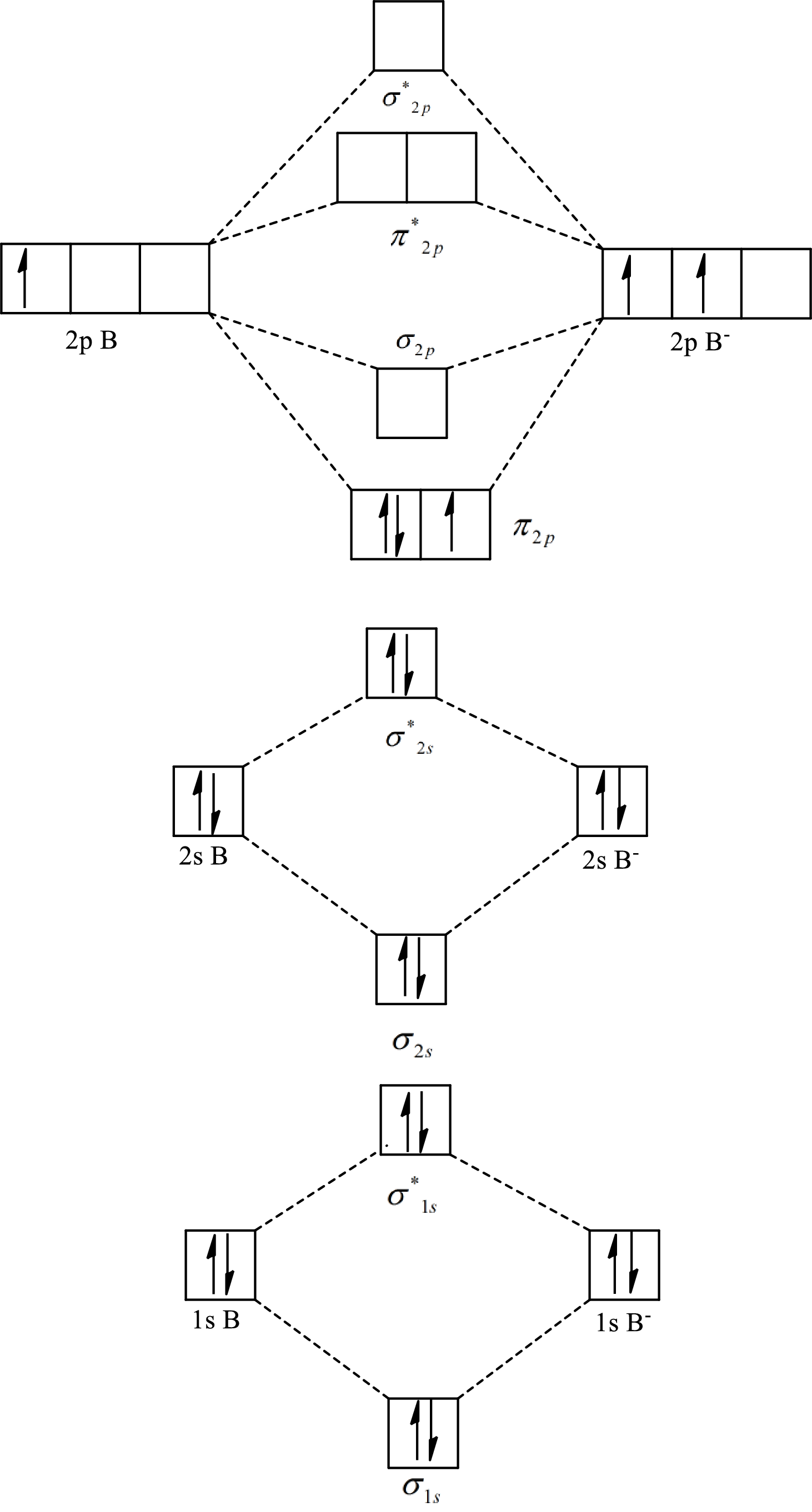
The formula to calculate the bond order is as follows:
Substitute 7 for the number of electrons in bonding orbitals and 4 for the number of electrons in antibonding orbitals in equation(1).
The molecular orbital diagram of
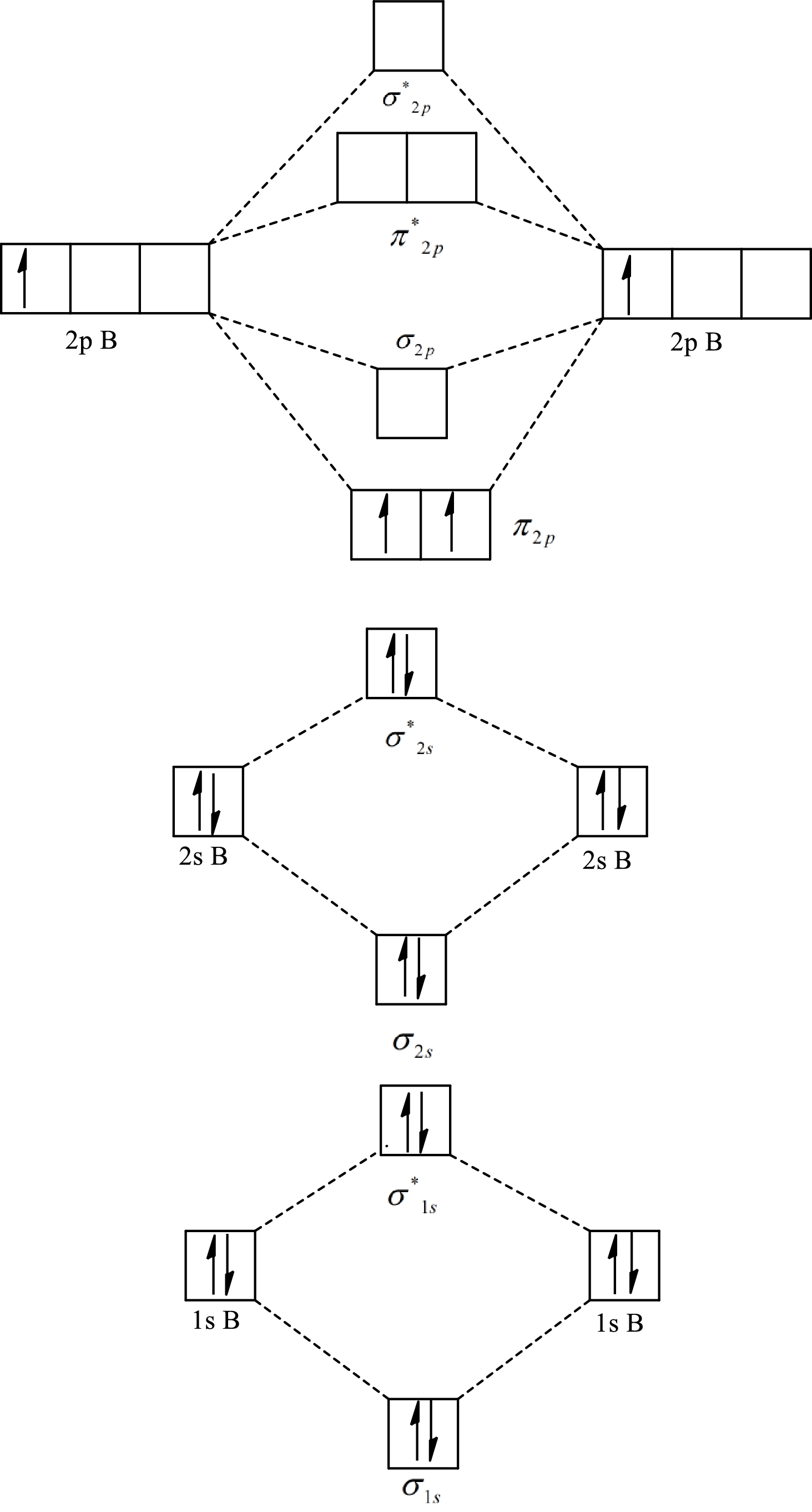
Substitute 6 for the number of electrons in bonding orbitals and 4 for the number of electrons in antibonding orbitals in equation(1).
The molecular orbital diagram of
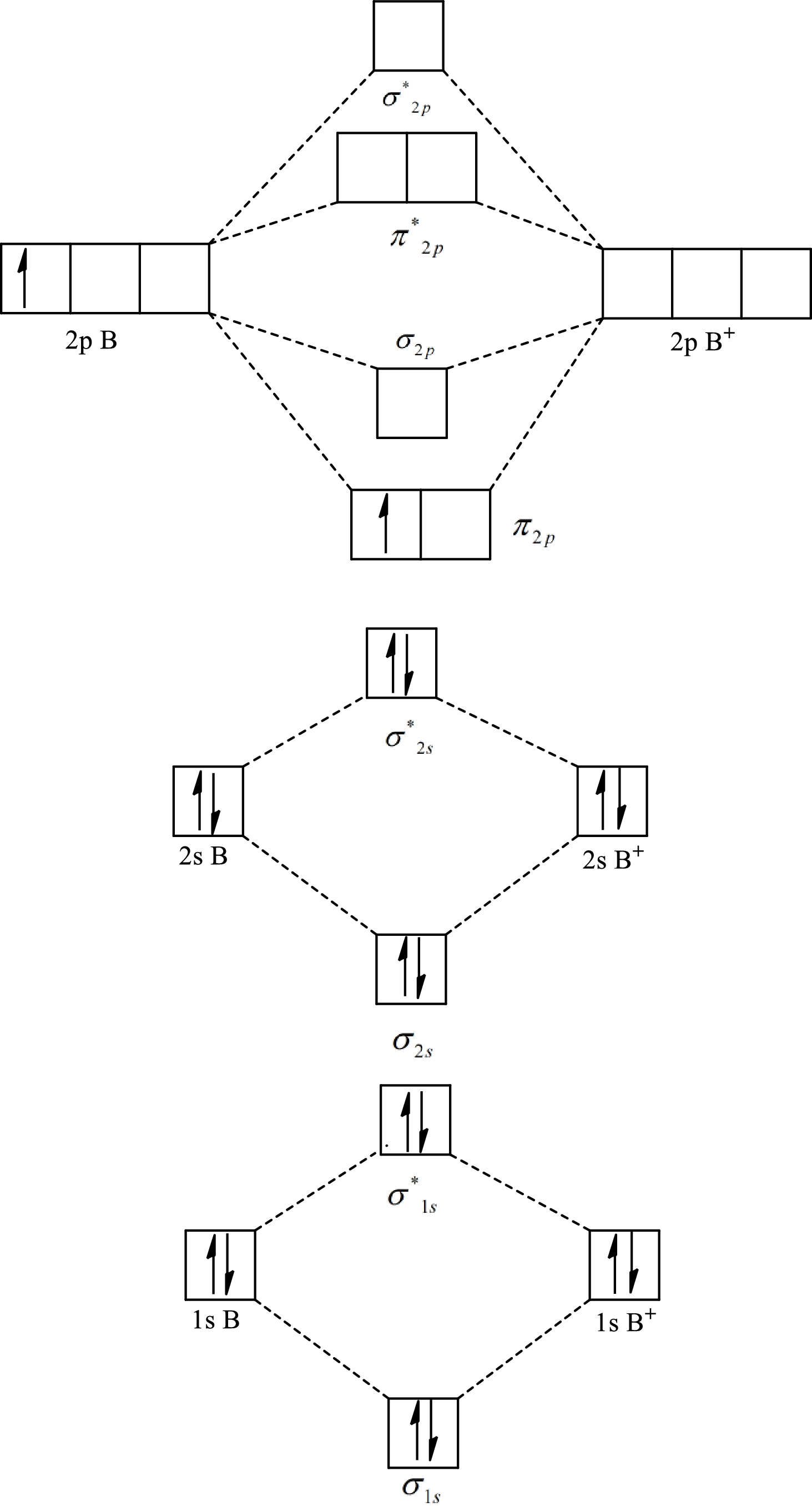
Substitute 5 for the number of electrons in bonding orbitals and 4 for the number of electrons in antibonding orbitals in equation(1).
Bond energy is directly proportional to the bond order. The highest bond order of
So the order of decreasing bond energy is
The order of decreasing bond energy of
(b)
Interpretation:
The order of decreasing bond length of
Concept introduction:
A molecular orbital diagram is a tool that is used to describe the chemical bonding formed between different molecules. It is used to predict the bond strength and the electronic transitions that a molecule can undergo.
(b)
Answer to Problem 11.37P
The order of the decreasing bond length is
Explanation of Solution
The molecular orbital diagram of
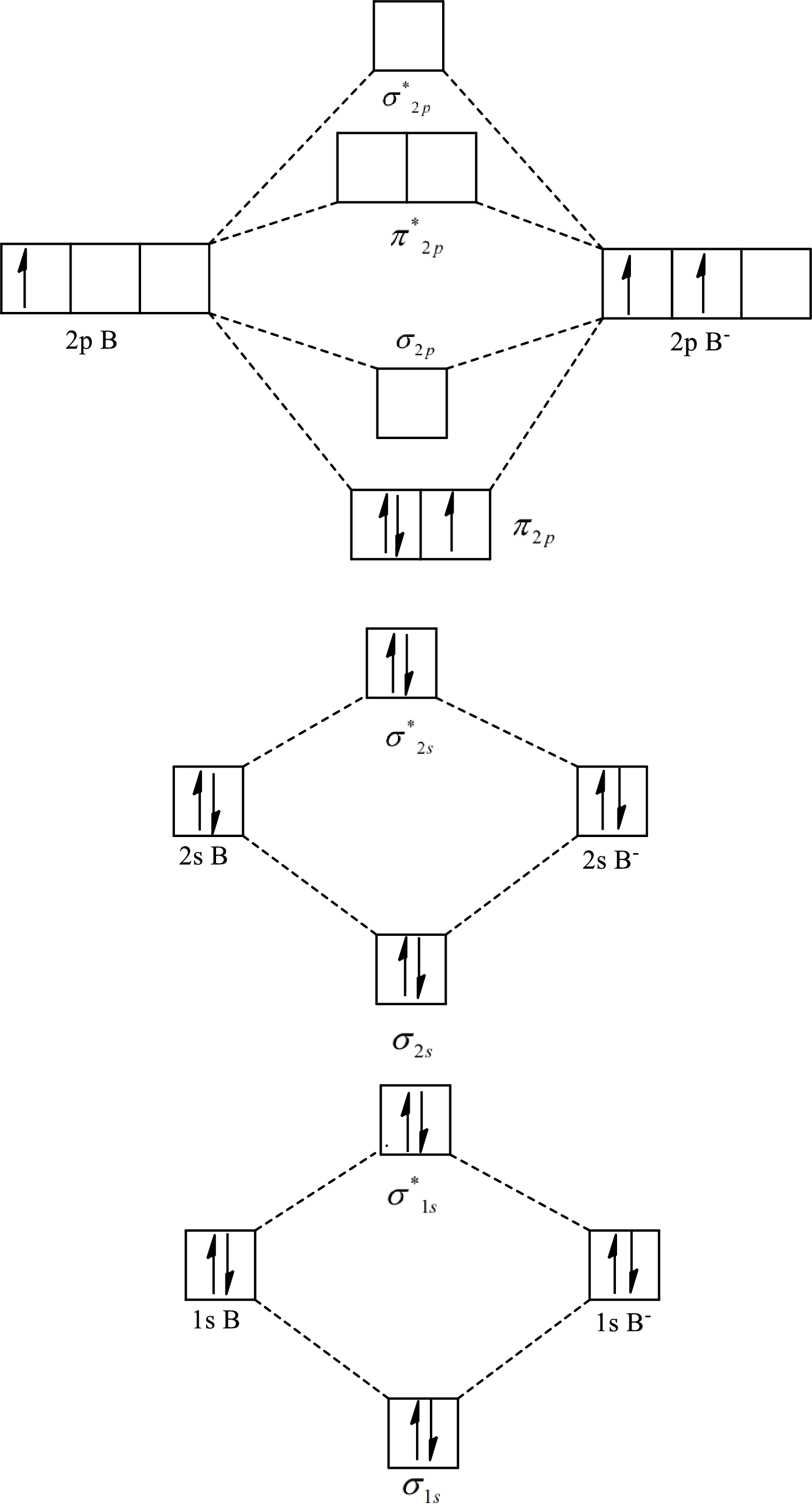
The formula to calculate the bond order is as follows:
Substitute 7 for the number of electrons in bonding orbitals and 4 for the number of electrons in antibonding orbitals in equation(1).
The molecular orbital diagram of
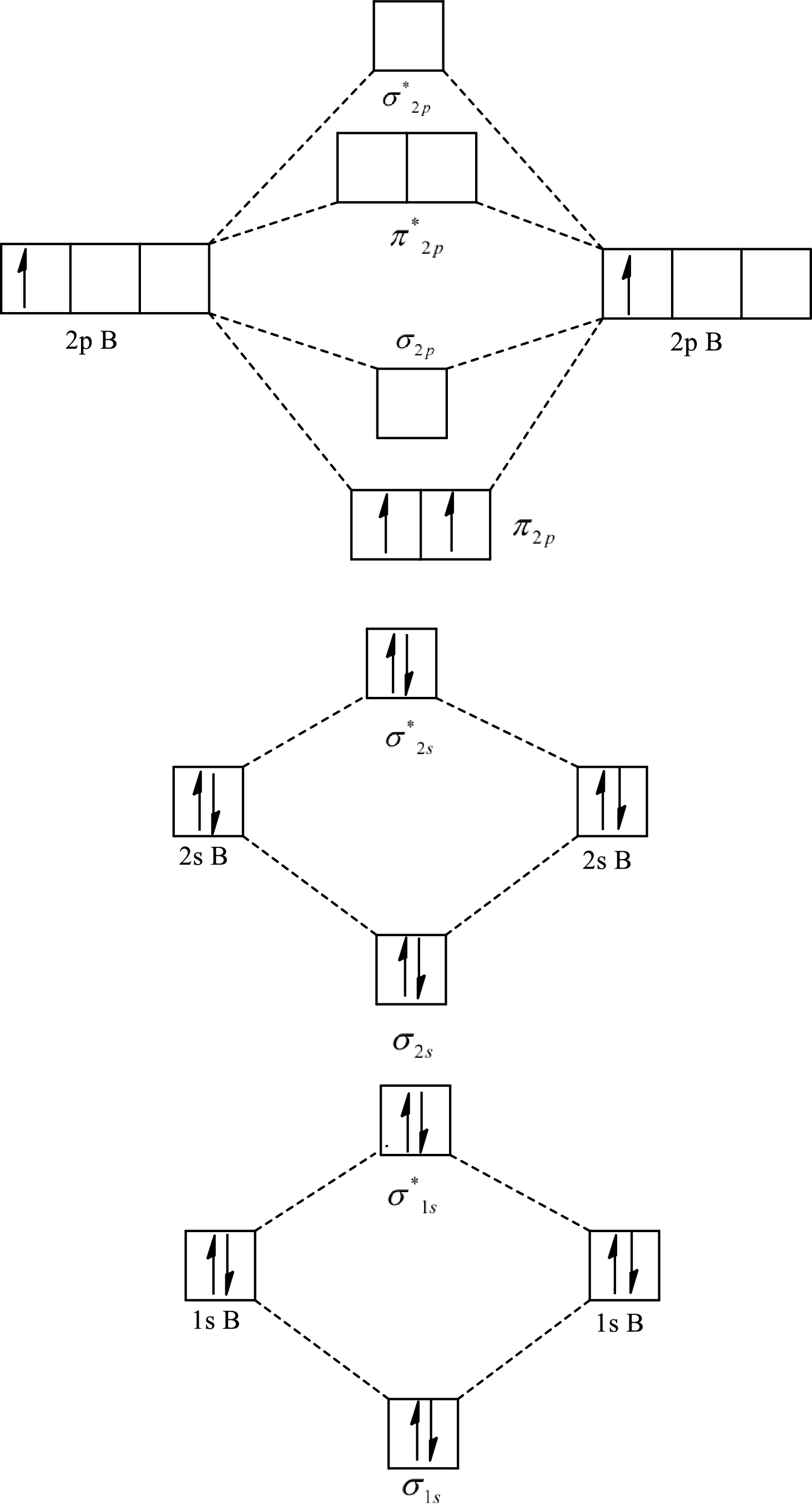
Substitute 6 for the number of electrons in bonding orbitals and 4 for the number of electrons in antibonding orbitals in equation(1).
The molecular orbital diagram of
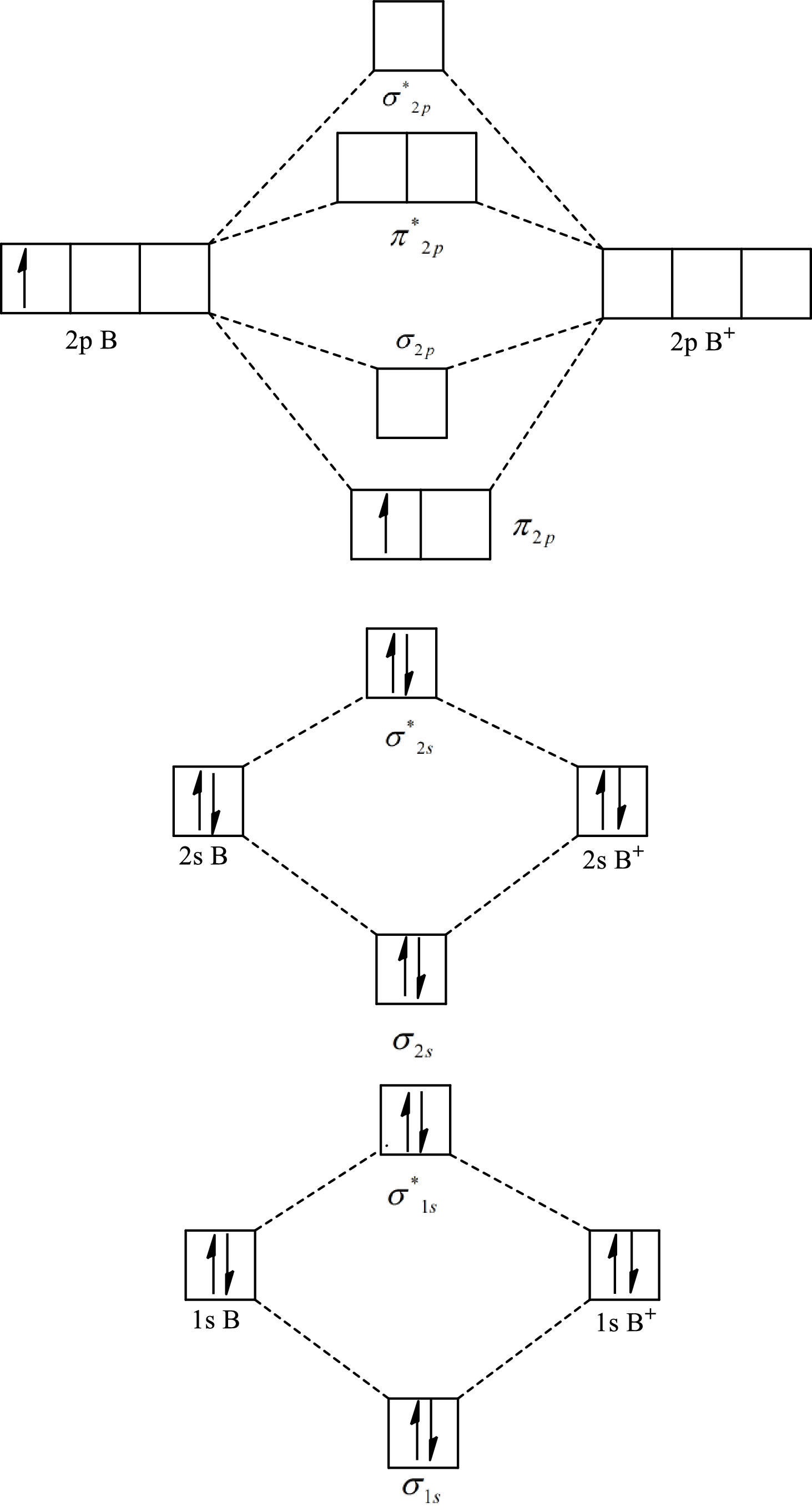
Substitute 5 for the number of electrons in bonding orbitals and 4 for the number of electrons in antibonding orbitals in equation(1).
Bond length decreases with the increase in bond energy. The highest bond order of
So the order of the decreasing bond length is
The order of the decreasing bond length is
Want to see more full solutions like this?
Chapter 11 Solutions
GEN CMB CHEM; CNCT+;ALEKS 360
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





