
(a)
Interpretation:
Given compound has to be named using
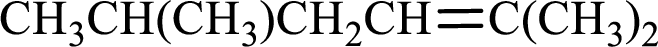
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming
There are about five rules that has to be followed for naming an alkene and an
- The longest continuous carbon chain in the compound that contains double bond or triple has to be identified. This is known as parent compound.
- Suffix “–ane” (in name of
alkane ) is replaced with “-ene” for alkene or “-yne” for alkyne. - Numbering has to be done so that the lowest number is given to the double or triple bond.
- Naming and numbering has to be given for each atom or group that is attached to the parent chain. Numbering has to be done in a way that substituents get the least numbering.
- If the alkenes have more than one double bond they are called as alkadienes (two double bonds) or alkatrienes (three double bonds). Appropriate suffix has to be used depending on the number of multiple bonds present in the compound.
(a)
Explanation of Solution
Given compound is,
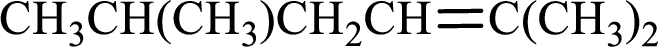
Longest carbon chain with double bond is found to contain five carbon atoms. Therefore, the parent alkane is hexane. As a double bond is present, the alkene name is hexene.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-2 and carbon-3. Therefore, the parent alkene is 2-hexene.
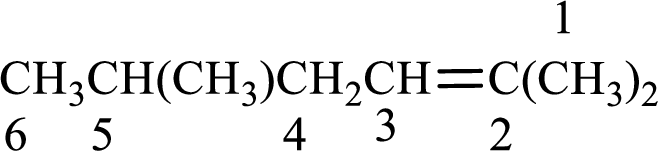
The substituent present in the given structure are a methyl group on carbon-5 and a methyl group on carbon-2. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkene as 2,5-dimethyl-2-hexene.
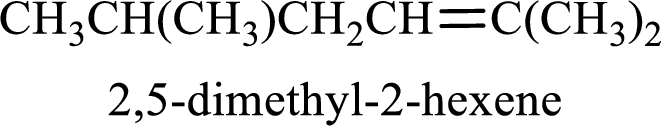
Longest carbon chain containing double bond is hexane. Position of double bond is 2-hexene. Substituents present in the chain are 2,5-dimethyl. IUPAC name of the alkene given is 2,5-dimethyl-2-hexene.
(b)
Interpretation:
Given compound has to be named using IUPAC nomenclature.

Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
Given compound is,

Longest carbon chain with triple bond is found to contain four carbon atoms. Therefore, the parent alkane is butane. As a triple bond is present, the alkyne name is butyne.
Numbering has to be given in a way that the triple bond gets the least numbering. In this case, triple bond is present between carbon-1 and carbon-2. Therefore, the parent alkyne is 1-butyne.
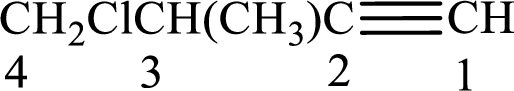
The substituents present in the given structure are a methyl group on carbon-3 and a chlorine atom on carbon-4. Substituents have to be arranged in alphabetical order. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkyne as 4-chloro-3-methyl-1-butyne.
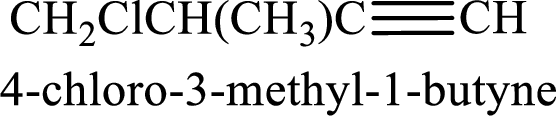
Longest carbon chain containing triple bond is butane. Position of triple bond is 1-butyne. Substituents present in the chain are 4-chloro-3-methyl. IUPAC name of the alkyne given is 4-chloro-3-methyl-1-butyne.
(c)
Interpretation:
Given compound has to be named using IUPAC nomenclature.
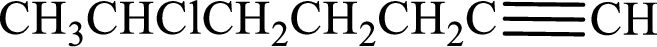
Concept Introduction:
Refer part (a).
(c)
Explanation of Solution
Given compound is,
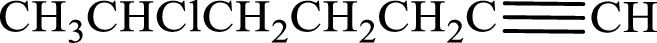
Longest carbon chain with triple bond is found to contain seven carbon atoms. Therefore, the parent alkane is heptane. As a triple bond is present, the alkyne name is heptyne.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-1 and carbon-2. Therefore, the parent alkene is 1-heptyne.
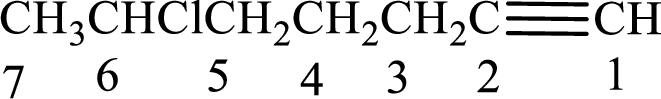
The substituent present in the given structure is a chlorine atom on carbon-6. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkyne as 6-chloro-1-heptyne.
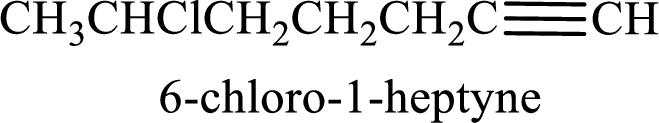
Longest carbon chain containing double bond is heptane. Position of triple bond is 1-heptyne. Substituents present in the chain are 6-chloro. IUPAC name of the alkyne given is 6-chloro-1-heptyne.
(d)
Interpretation:
Given compound has to be named using IUPAC nomenclature.
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming cycloalkenes:
- The number of carbon atoms present in the ring is counted and the name of the alkane that has the same number of carbon atoms is given by adding prefix “cyclo-” to the alkane name. Suffix “-ane” is changed as “-ene”.
- The double bond that is present in the ring is given always the number 1.
- If the ring is substituted, then the names of the group or atoms have to be placed before the name of cycloalkene.
- If the ring contains more than one substituent, then the numbers has to be used in a way that it gives the lowest position for the substituents following position 1 for the double bond.
(d)
Explanation of Solution
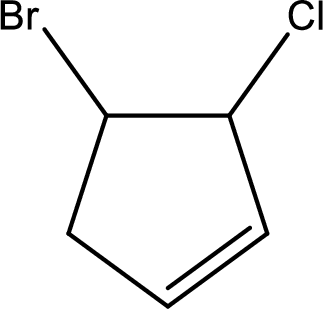
Given compound is,
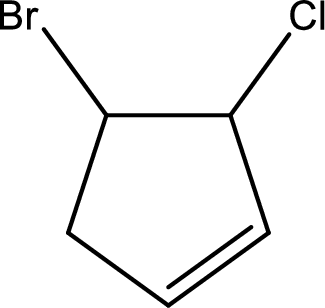
Given compound is found to contain five carbon atoms in a cyclic ring structure. Therefore, the parent cycloalkane is cyclopentane. As there is a double bond present in the ring, the parent compound name is cyclopentene.
Numbering has to be given in a way that the double bond gets the least numbering and also the substituents. In this case, double bond is present between carbon-1 and carbon-2.
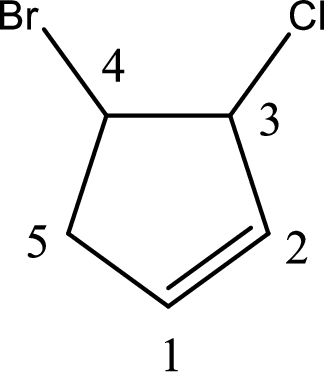
The substituents present in the given structure are a chlorine atom on carbon-3 and a bromine atom on carbon-4. Substituents have to be arranged in alphabetical order. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of compound as 4-bromo-3-chlorocyclopentene.
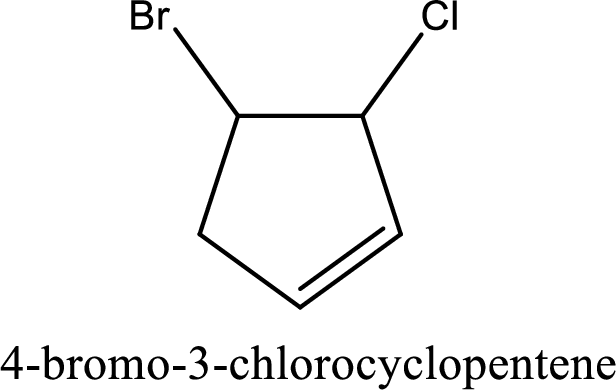
Parent chain is found to be cyclopentene. Position of double bond is carbon-1. Substituent present in the chain is 4-bromo-3-chloro. IUPAC name of the compound given is 4-bromo-3-chlorocyclopentene.
Want to see more full solutions like this?
Chapter 11 Solutions
Connect 2-Year Online Access for General, Organic, and Biochemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





