
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
9th Edition
ISBN: 9781337598255
Author: Spencer L. Seager
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.52E
Which of the following cycloalkanes could show geometric isomerism? For each that could, draw structural formulas, and name both the cis- and the trans- isomers.
a. 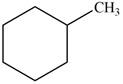 c.
c. 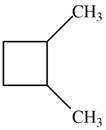
b. 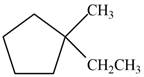 d.
d. 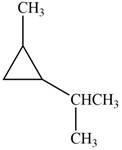
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1. a. Draw and name the five cycloalkane structures of formula C5H10. Can any of these structures give rise to geometric (cis-trans) isomerism? If so, show the cis and trans stereoisomers.
b. Draw and name the eight cycloalkane structures of formula C6H12 that do not show geometric isomerism.
c. Draw and name the four cycloalkanes of formula C6H12 that do have cis-trans isomers.
2. Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description.
(a) an isopropylheptane (b) a diethyldecane (c) a cis-diethylcyclohexane
(d) a trans-dihalocyclopentane (e) a (2,3-dimethylpentyl)cycloalkane (f) a bicyclononane
3. 2. refer to the photo attached and answer the ff.3-33, 3-34
Q1. Name each of the following structures by the
IUPAC system?
B. W
C.
Q2. Write the structural formula for the following?
A. 1,4-dichloro-2-pentene
B. 2-bromo-1,3-pentadiene
C. vinyl cyclohexane
Q3. Which of the following compounds can exist as cis-
trans isomers? Draw their structures?
A.. pentene
B. 2-methyl-2-pentene
Q3. Writ an equitation for the
A. Reaction for bromine at room temperature
1. Propene
B. The acid catalyzed addition of water to
1. 3-hexene
Q4. For the addition of HBr to 3-methyl-1-butene, two
products were observed. Show the reaction mechanism
to explain the formation of both products.
HBr -
3-methyl-1-butene
major
minor
Determine whether cis-trans isomerism is probable for the following cycloalkanes. If it is possible, draw structural formulas for the cis and trans isomers.
a. 1-ethyl-1-methylcyclopentane
b. ethylcyclohexane
c. 1,4-diethylcyclohexane
d. 1,1-dimethylcyclooctane
e. methylcycloheptane
Chapter 11 Solutions
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
Ch. 11 - Prob. 11.1ECh. 11 - Prob. 11.2ECh. 11 - Prob. 11.3ECh. 11 - Prob. 11.4ECh. 11 - Prob. 11.5ECh. 11 - Prob. 11.6ECh. 11 - Prob. 11.7ECh. 11 - Prob. 11.8ECh. 11 - Prob. 11.9ECh. 11 - Prob. 11.10E
Ch. 11 - Prob. 11.11ECh. 11 - Prob. 11.12ECh. 11 - Prob. 11.13ECh. 11 - Prob. 11.14ECh. 11 - What molecular geometry exists when a central...Ch. 11 - Compare the shapes of unhybridized p and...Ch. 11 - Use Example 11.1 and Tables 11.2 and 11.6 to...Ch. 11 - Prob. 11.18ECh. 11 - Prob. 11.19ECh. 11 - Prob. 11.20ECh. 11 - Prob. 11.21ECh. 11 - Prob. 11.22ECh. 11 - Prob. 11.23ECh. 11 - Write a condensed structural formula for the...Ch. 11 - Write a condensed structural formula for the...Ch. 11 - Write an expanded structural formula for the...Ch. 11 - Prob. 11.27ECh. 11 - Classify each of the following compounds as a...Ch. 11 - Why are different conformations of an alkane not...Ch. 11 - Which of the following pairs represent structural...Ch. 11 - Prob. 11.31ECh. 11 - Prob. 11.32ECh. 11 - Identify the following alkyl groups: a. b....Ch. 11 - Prob. 11.34ECh. 11 - Prob. 11.35ECh. 11 - Draw a condensed structural formula for each of...Ch. 11 - Prob. 11.37ECh. 11 - Prob. 11.38ECh. 11 - Prob. 11.39ECh. 11 - Prob. 11.40ECh. 11 - The following names are incorrect, according to...Ch. 11 - The following names are incorrect, according to...Ch. 11 - Prob. 11.43ECh. 11 - Write the correct IUPAC name for each of the...Ch. 11 - Write the correct IUPAC name for each of the...Ch. 11 - Draw the structural formulas corresponding to each...Ch. 11 - Prob. 11.47ECh. 11 - Which of the following pairs of cycloalkanes...Ch. 11 - Prob. 11.49ECh. 11 - Prob. 11.50ECh. 11 - Prob. 11.51ECh. 11 - Which of the following cycloalkanes could show...Ch. 11 - Prob. 11.53ECh. 11 - Using the prefix cis- or trans-, name each of the...Ch. 11 - Prob. 11.55ECh. 11 - The compound decane is a straight-chain alkane....Ch. 11 - Explain why alkanes of low molecular weight have...Ch. 11 - Suppose you have a sample of 2-methylhexane and a...Ch. 11 - Identify circle the alkanelike portions of the...Ch. 11 - Prob. 11.60ECh. 11 - Prob. 11.61ECh. 11 - Write a balanced equation for the incomplete...Ch. 11 - Prob. 11.63ECh. 11 - Prob. 11.64ECh. 11 - Prob. 11.65ECh. 11 - Prob. 11.66ECh. 11 - Prob. 11.67ECh. 11 - Prob. 11.68ECh. 11 - Would you expect a molecule of urea produced in...Ch. 11 - Prob. 11.70ECh. 11 - Prob. 11.71ECh. 11 - Prob. 11.72ECh. 11 - Prob. 11.73ECh. 11 - Prob. 11.74ECh. 11 - Prob. 11.75ECh. 11 - A semi-truck loaded with cyclohexane overturns...Ch. 11 - Prob. 11.77ECh. 11 - Oil spills along coastal shores can be disastrous...Ch. 11 - Prob. 11.79ECh. 11 - Prob. 11.80ECh. 11 - Use the generic formula for alkanes (CnH2n+2) to...Ch. 11 - Prob. 11.82ECh. 11 - Which of the following is an example of an alkane?...Ch. 11 - Prob. 11.84ECh. 11 - Prob. 11.85ECh. 11 - Prob. 11.86ECh. 11 - The deadly property of carbon monoxide, if...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Distinguish between isomerism and resonance. Distinguish between structural and geometric isomerism. When writing the various structural isomers, the most difficult task is identifying which are different isomers and which are identical to a previously written structurethat is, which are compounds that differ only by the rotation of a carbon single bond. How do you distinguish between structural isomers and those that are identical? Alkenes and cycloalkanes are structural isomers of each other. Give an example of each using C4H8. Another common feature of alkenes and cycloalkanes is that both have restricted rotation about one or more bonds in the compound, so both can exhibit cis- trans isomerism. What is required for an alkene or cycloalkane to exhibit cis-trans isomerism? Explain the difference between cis and trans isomers. Alcohols and ethers are structural isomers of each other, as are aldehydes and ketones. Give an example of each to illustrate. Which functional group in Table 21-4 can be structural isomers of carboxylic acids? What is optical isomerism? What do you look for to determine whether an organic compound exhibits optical isomerism? 1-Bromo-1-chloroethane is optically active whereas 1-bromo-2-chloroethane is not optically active. Explain.arrow_forwardHow does the structure of a cycloalkane differ from that of a straight-chain or branched-chain alkane?arrow_forwardIs the general formula of a cycloalkanes the same as the general formula of an alkane, CnH2n+2? Draw any structural diagram to illustrate your answer.arrow_forward
- Summarize the nomenclature rules for alkanes, alkenes, alkynes, and aromatic compounds. Correct the following false statements regarding nomenclature of hydrocarbons. a. The root name for a hydrocarbon is based on the shortest continuous chain of carbon atoms. b. The suffix used to name all hydrocarbons is -ane. c. Substituent groups are numbered so as to give the largest numbers possible. d. No number is required to indicate the positions of double or triple bonds in alkenes and alkynes. e. Substituent groups get the lowest number possible in alkenes and alkynes. f. The ortho- term in aromatic hydrocarbons indicates the presence of two substituent groups bonded to carbon- 1 and carbon-3 in benzene.arrow_forwardWhat is the difference in bonding and in general molecular formula between an alkene and a cycloalkane with the same number of carbon atoms?arrow_forwardExplain why two different straight-chain alkanes could not be constitutional isomers.arrow_forward
- Consider a sample of a hydrocarbon at 0.959 atm and 298 K. Upon combusting the entire sample in oxygen, you collect a mixture of gaseous carbon dioxide and water vapor at 1.51 atm and 375 K. This mixture has a density of 1.391 g/L and occupies a volume four times as large as that of the pure hydrocarbon. Determine the molecular formula of the hydrocarbon and name it.arrow_forwardWhat is the difference in bonding and in the general molecular formula between an alkene and an alkane with the same number of carbon atoms?arrow_forwardWhat is meant by the term “unsaturated hydrocarbon”? What structural feature characterizes unsaturated hydrocarbons?arrow_forward
- . Alkenes and alkynes are characterized by their ability to undergo rapid, complete reactions, by which other atoms attach themselves to the carbon atoms of the double or triple bond.arrow_forward2. Determine whether cis-trans isomerism is possible for each of the following cycloalkanes. If so, then draw structural formulas for the cis and trans isomers.. a. Methylcyclohexane b. 1,1-Dimethylcyclohexane c. 1,3-Dimethylcyclobutane d. 1-Ethyl-2-methylcyclobutanearrow_forward1. Name and draw the isomers form from the given molecular formula. a. C₂H16 b. C4H,Br₂ 2. Draw the structure of each of the following cycloalkanes a. 1-Bromo-2-methylcyclobutane b. 1,2-Dibromo-3-methylcyclohexanearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License