
(a)
Interpretation:
Whether the reaction of ethylene with
Concept introduction:
The reaction of
Answer to Problem 11.53AP
The reaction of ethylene with
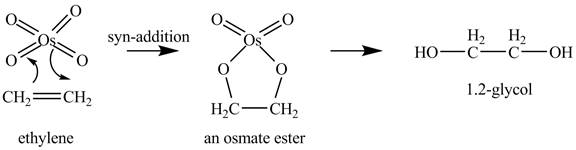
Explanation of Solution
Ethylene reacts with

Figure 1
Since, the product
The reaction of ethylene with
(b)
Interpretation:
Whether the reaction of
Concept introduction:
The reaction of alkenes with
Answer to Problem 11.53AP
The reaction of
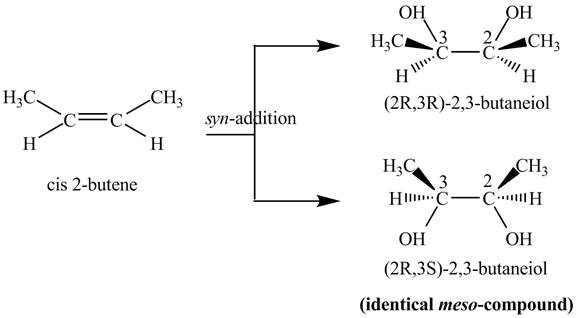
Explanation of Solution
The compound
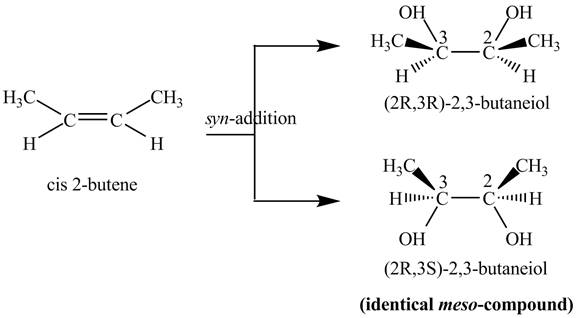
Figure 2
The two products of
The reaction of
(c)
Interpretation:
Whether the reaction of
Concept introduction:
The reaction of alkenes with
Answer to Problem 11.53AP
The reaction of
Explanation of Solution
The compound
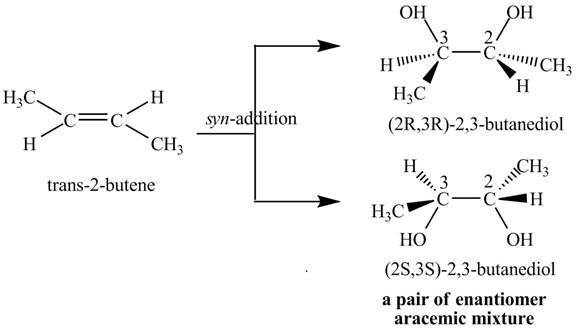
Figure 3
The products
The reaction of
(d)
Interpretation:
Whether the reaction of
Concept introduction:
The reaction of alkenes with
Answer to Problem 11.53AP
The reaction of
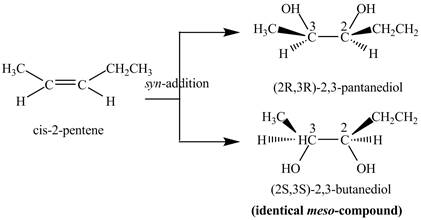
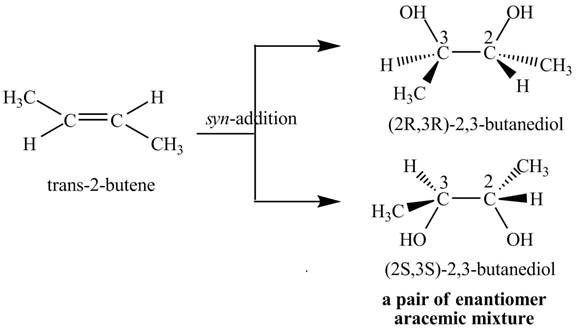
Explanation of Solution
The reaction of
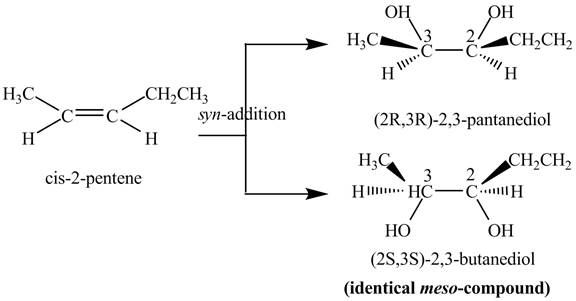
Figure 4
The two products:
The reaction of
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Write the mechanism of the hydrolysis of cis-5, 6-epoxydecane by reaction with aqueous acid. What is the stereochemistry of the product, assuming normal backside SN2 attack?arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardWrite out all the isomers of the compound with molecular formula C4H10O . Select the normal/ primaryisomer and treat it with conc. H2SO4 and heat. Identify the reaction and give the product ‘A’ from it. 2. When ‘A’ is treated with HBr in the presence of a peroxide, give the name and structure of the product.arrow_forward
- 3b)Give the mechanisms for the following transformations:arrow_forwardCompound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct. Can be more than one answerarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

