
Concept explainers
(a)
Interpretation:
The product of the reaction of dibutyl sulfide with
Concept introduction:
Hydrogen perioxide
Sulfur containing organic compounds has many similar chemical properties as oxygen contain compound such as ether and aclcohol.
Answer to Problem 11.45AP
The product of the reaction of dibutyl sulfide with

Explanation of Solution
The dibutyl sulfide undergoes an oxidation reaction with hydrogen peroxide. The dibutyl sulfide reacts with
The corresponding

Figure 1
The product of the reaction of dibutyl sulfide with
(b)
Interpretation:
The product of the reaction of dibutyl sulfide with
Concept introduction:
Hydrogen perioxide
Sulfur containing organic compounds has many similar chemical properties as oxygen contain compound such as ether and aclcohol.
Answer to Problem 11.45AP
The product of the reaction of dibutyl sulfide with
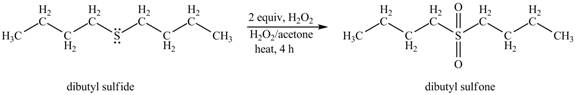
Explanation of Solution
The dibutyl sulfide undergoes an oxidation reaction with hydrogen peroxide. The dibutyl sulfide reacts with
The corresponding chemical reaction is shown below.
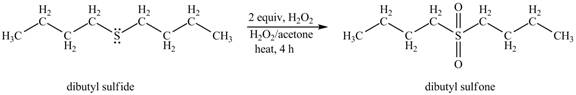
Figure 2
The product of the reaction of dibutyl sulfide with
(c)
Interpretation:
The product of the reaction of
Concept introduction:
The magnesium monoperoxyphthalate
Answer to Problem 11.45AP
The product of the reaction of
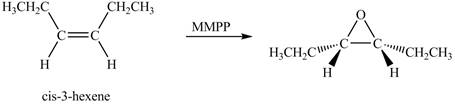
Explanation of Solution
The compound
The corresponding chemical reaction is shown below.
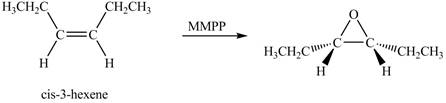
Figure 3
The product of the reaction of
(d)
Interpretation:
The product of the reaction of given compound with
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.45AP
The product of the reaction of given compound with
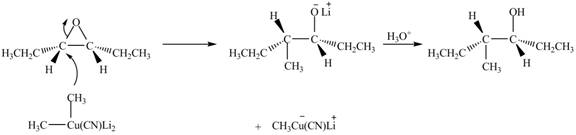
Explanation of Solution
The epoxide undergoes ring-opening reaction in the presence of acid. The dilithium dimethylcyanocuprate molecule generates a nucleophile
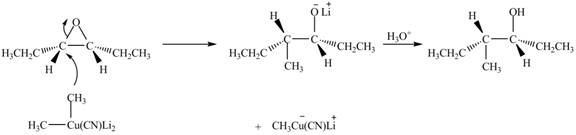
Figure 4
The product of the reaction of given compound with
(e)
Interpretation:
The product of the reaction of given compound with solvent
Concept introduction:
Epoxides undergo nucleophilic ring-opening reactions which are acid-catalyzed. If the epoxide is unsymmetrical, then the anionic nucleophile will attack the less-hindered carbon atom of the ring. If the reaction conditions are acidic, then the reaction will occur at the more substituted carbon atom.
Answer to Problem 11.45AP
The product of the reaction of given compound with solvent
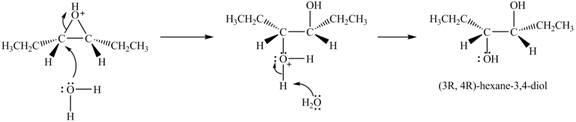
Explanation of Solution
The epoxide undergoes ring opening reaction in the presence of acid. The water molecule acts a nucleophile and attacks on the more substituted carbon atom of the epoxide ring to from

Figure 5
The product of the reaction of given compound with solvent
(f)
Interpretation:
The product of the reaction of given compound with periodic acid is to be predicated.
Concept introduction:
The periodic acid acts as a strong oxidizing agent. The periodic acid reacts with a vicinal diol to form two
Answer to Problem 11.45AP
The product of the reaction of given compound with periodic acid is shown below.
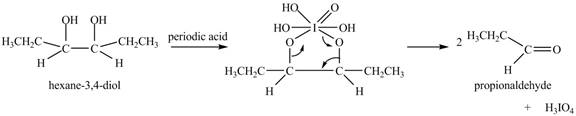
Explanation of Solution
The given compound is vicinal diol. It reacts with periodic acid to form two aldehydes. The carbon-carbon bond between the carbon atoms attached to two adjacent hydroxyl groups gets breaks. The corresponding chemical reaction is shown below.

Figure 6
The product of the reaction of given compound with periodic acid is shown in Figure 6.
(g)
Interpretation:
The product of the reaction of given compound with
Concept introduction:
The metal hydride reagents are good reducing agents such as
Answer to Problem 11.45AP
The product of the reaction of given compound with
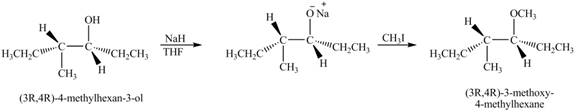
Explanation of Solution
The base
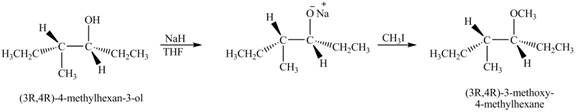
Figure 7
The product of the reaction of given compound with
(h)
Interpretation:
The product of the reaction of
Concept introduction:
Oxymercuration reaction is a type of reaction in which an alkene get converted into an alcohol. The mercuric acetate is used in the reaction as reagent. This reagent attacks the alkene to form a cyclic intermediate compound which further undergoes reduction to form alcohol.
Answer to Problem 11.45AP
The product of the reaction of
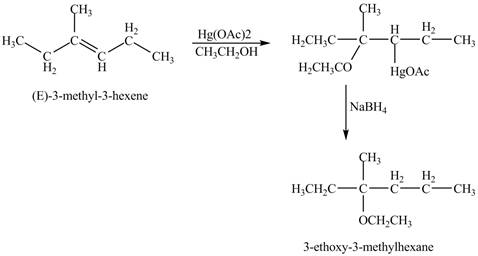
Explanation of Solution
The mercuric acetate
The corresponding chemical reaction is shown below.
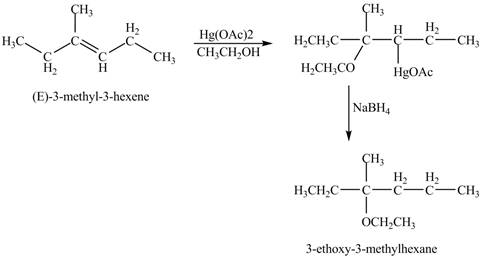
Figure 8
The product of the reaction of
(i)
Interpretation:
The product of the reaction of the given compound with acidic methanol is to be predicated.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 11.45AP
The product of the reaction of the given compound with acidic methanol is shown below.
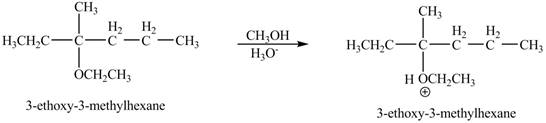
Explanation of Solution
The proton of the acid will protionate the ether. The protonated ether will increase the electrophilic characters of carbon atom attached to the oxygen atom. The methonal can acts as nuclephile, however the nucleophilic charater of methoxy-group and ethoxy group is similar. Therefore, no further reaction will take place.
The corresponding chemical reaction is shown below.
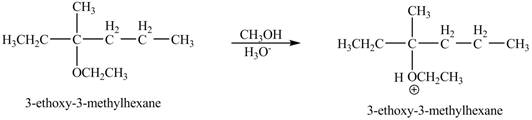
Figure 9
The product of the reaction of the given compound with acidic methanol is shown in Figure 9.
(j)
Interpretation:
The product of the reaction of
Concept introduction:
Grignard reagents are
Answer to Problem 11.45AP
The product of the reaction of
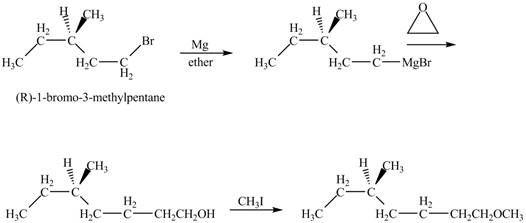
Explanation of Solution
The alkyl halide will react with magnisum metal to from gridnard reagent. These Grignard reagent reacts with epoixde to form alcohol. The alcohol reacts with
The corresponding chemical reaction is shown below.
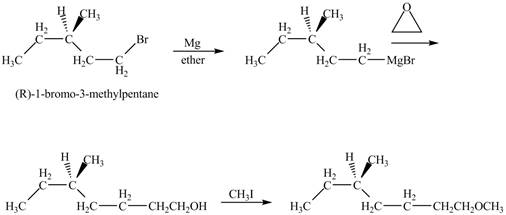
Figure 10
The product of the reaction of
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Wolff-Kishner reduction of compound W gave compound A. Treatment of A with m-chloroperbenzoic acid gave B which on reduction with LiAH4 gave C. Oxidation of compound C with chromic acid gave D (C9H14O). Suggest the structures for A, B, C, and D.arrow_forwardCompound A, C3H7Br, does not react with cold dilute potassium permanganate solution. Upon treatment with potassium hydroxide in ethanol, A gives only product B, C3H6. Unlike A, B decolourises potassium permanganate solution. Ozonolysis of Bgives C, C2H4O, and D, CH2O. Suggest the structural formulae of A, B, C and D.Write the equations for all the reactions involved.arrow_forwardCompound A(C10H12O)gives off oxygen on treatment with sodium metal and also decolorizes Br2 in CCl4 to give organic compound B. Compound A on treatment with I2 in NaOH gives iodoform and salt C which after acidification gives a white solid D(C7H6O2). Using knowledge of organic chemistry identify structures A,B,C and Darrow_forward
- Represent the reaction equation of the alkene supplied to you with propanol, in acidic medium, indicating thestereochemistry of all the products formed.arrow_forward3. Obtain acetophenone and acetaldehyde by reaction of glycols with periodic acid. Justify your answer with the reaction mechanism.arrow_forwardTwo isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. Which of the following isomeric pairs best match this data?arrow_forward
- Two isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. What is the isometric pair of A and B that corresponds?arrow_forwardA hydrocarbon (X), with the molecular formula: C8H14 is reduced in presence of sodium and liquid ammonia to give the only product (Y) with the molecular formula: C8H16. Compounds X and Y both resulting 2,5-dimethylhexane when treated with hydrogen and platinum catalyst (H2/Pt). As a result of the oxidative cleavage of compound Y (by using KMnO4 / H2SO4), a single carboxylic acid derivative with C4H8O2 molecular formula is formed. Again, as a result of the reaction of Y with perbenzoic acid, the chiral compound C8H14O is observed, but the reaction of compound Y with bromine gives the achiral C8H14Br2 as the product.arrow_forwardCompound P (C2H4) which is an alkene undergoes reaction with HCl to produce compound Q (C2H5Cl). Reaction of compound Q with benzene in the presence of AlCl3 as catalyst produces compound R. Then, nitration of compound R in the presence ofnH2SO4 produces two compounds, S and T. But when compound R is reacted with a hot acidified solution of alkaline KMnO4 gives compound U. Deduce the structure of compounds P to U.arrow_forward
- Birch reduction of 2-methoxynaphthalene gave a mixture of two isomeric compounds, each having the molecular formula C11H14O. Suggest reasonable structures for these compounds.arrow_forwardHydrocarbon A has the formula C9H12 and absorbs 3 equivalents of H2 to yield B, C9H18, when hydrogenated over a Pd/C catalyst. On treatment of A with aqueous H2SO4 in the presence of mercury(II), two isomeric ketones, C and D, are produced . Oxidation of A with KMnO4 gives a mixture of acetic acid (CH3COOH) and the tricarboxylic acid E. Propose structures for compounds A-D, and write the reactions.arrow_forwardGive the major organic product(s) or reagents needed for the following reactions or sequences of reactions. Show all relevant stereochemistry.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





