
Concept explainers
(a)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the
Answer to Problem 11.77AP
The curved arrow mechanism for the reaction is shown below.
Explanation of Solution
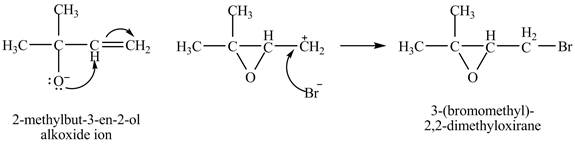
The given reaction is shown below.
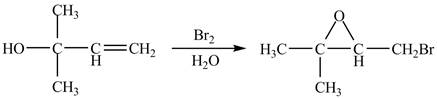
Figure 1
The oxygen atom of the alkoxide ion attack carbon of double bond to form

Figure 2
The curved arrow mechanism for given reaction is shown in Figure 2.
(b)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
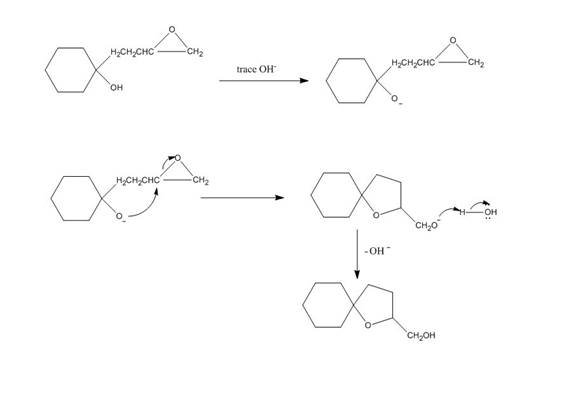
The given reaction is shown below.
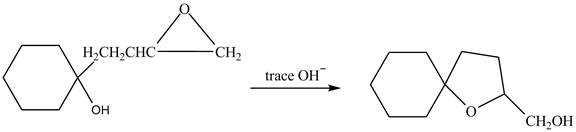
Figure 3
Hydroxide ion abstract the proton from hydroxyl group of given compound. The oxide attacks the carbon of epoxide ring to form a five membered ring. The alkoxide ion formed is protonated in presence of water. The curved arrow mechanism of the given reaction is shown below.
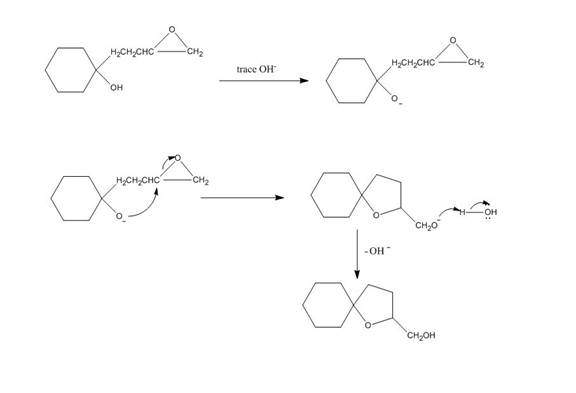
Figure 4
The curved arrow mechanism for given reaction is shown in Figure 4.
(c)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
Explanation of Solution
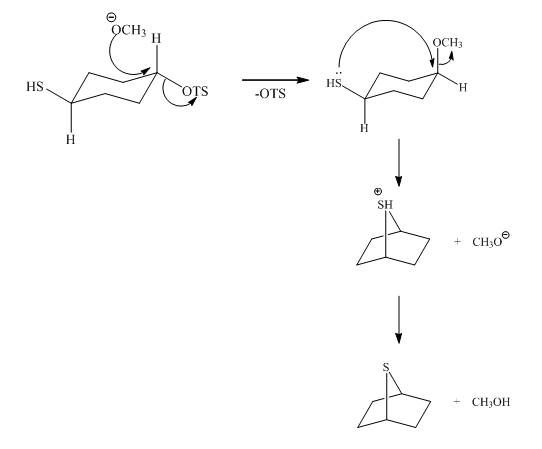
The given reaction is shown below.
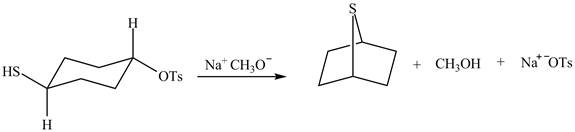
Figure 5
The methoxy group attacks the carbon containing sulfonate ester group. The nucleophilic displacement reaction occurs. The sulfonate ester group is displaced by methoxy group. Then neighbouring group participation of sulfur atom displaces the methoxy group. The sulfonium ion formed undergoes deprotonation to form the product. The curved arrow mechanism of the given reaction is shown below.

Figure 6
The curved arrow mechanism for given reaction is shown in Figure 6.
(d)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
Explanation of Solution
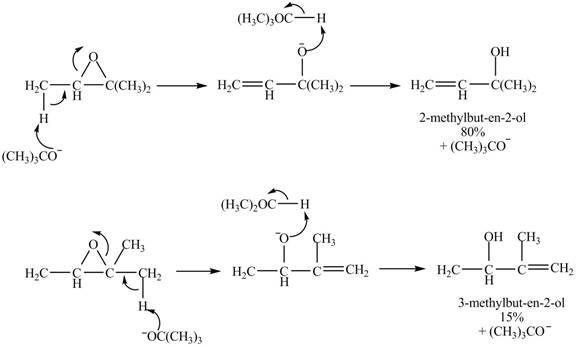
The given reaction is shown below.
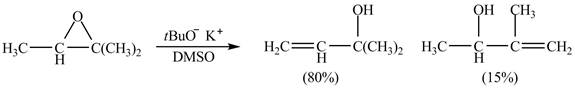
Figure 7
A strong base is used in the reaction; hence elimination reaction will take place. The given compound contains two
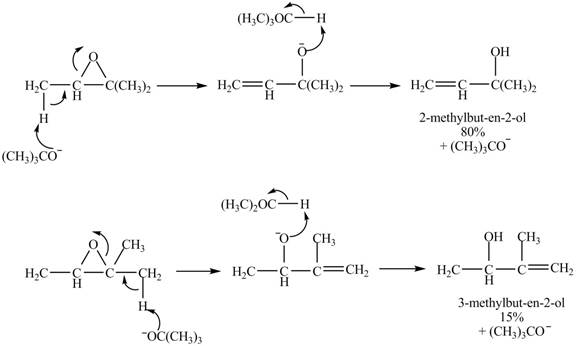
Figure 8
The curved arrow mechanism for given reaction is shown in Figure 8.
(e)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
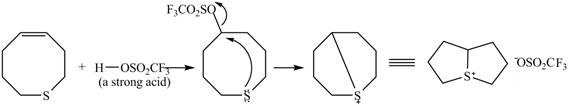
Explanation of Solution
The given reaction is shown below.
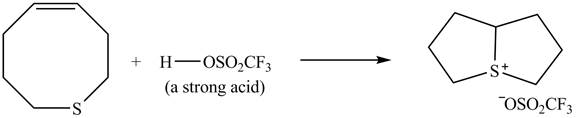
Figure 9
The
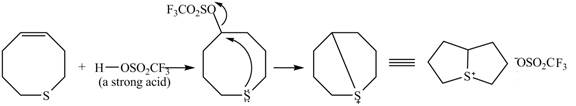
Figure 10
The curved arrow mechanism for given reaction is shown in Figure 10.
(f)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
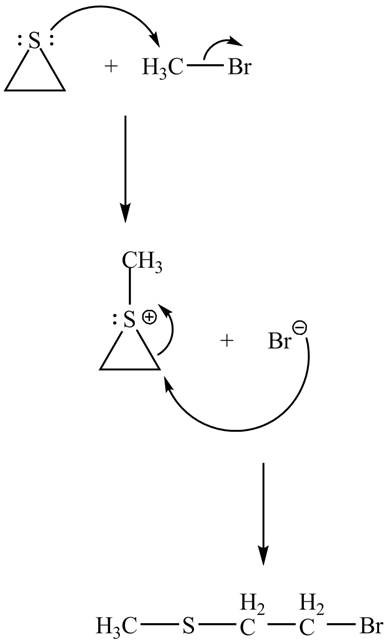
Explanation of Solution
The given reaction is shown below.
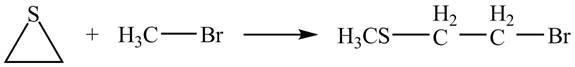
Figure 11
The lone pair of sulphur atom attacks the carbon of methyl bromide. The sulfur atom of the ring gets methylated. Then bromide ion attacks the carbon atom of ring which results in ring opening to form the desired product. The curved arrow mechanism of the given reaction is shown below.

Figure 12
The curved arrow mechanism for given reaction is shown in Figure 12.
(g)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
Explanation of Solution
The given reaction is shown below.
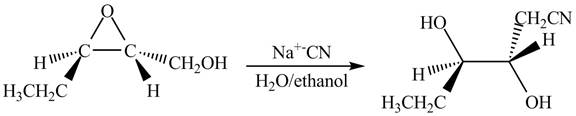
Figure 13
The neighbouring group participation of oxygen atom takes place in the given reaction. The oxygen atom of hydroxyl group attacks the electrophilic carbon atom of the epoxide ring which results in the formation of new epoxide ring. Then cyanide ion attacks at the electrophilic carbon of epoxide which results in ring opening. The oxide ion takes proton form ethanol to form desired product. The curved arrow mechanism for given reaction is shown below.

Figure 14
The curved arrow mechanism for given reaction is shown in Figure 14.
(h)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
Neighbouring group participation is -the interaction of the lone pair electrons of an atom (hetero) or electron present in sigma and pi bond with the parent molecule to increase the speed of the reaction. It is also known as anchimeric assistance.
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
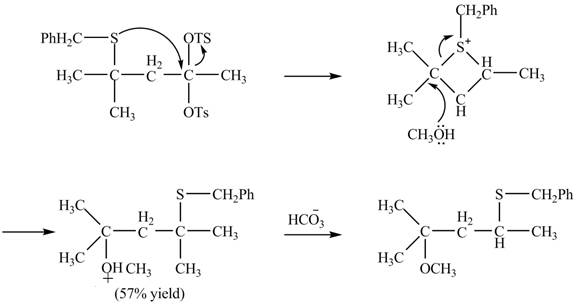
Explanation of Solution
The given reaction is shown below.
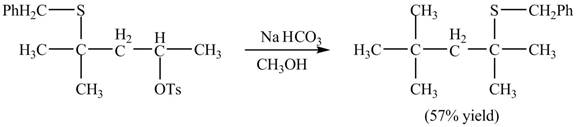
Figure 15
The sulfur atom attacks at the electrophilic carbon atom to form a four membered ring. The nucleophilic attack by methanol at the ring results in ring opening. Then the base abstracts proton from the protonated hydroxyl group to form the desired product. The curved-arrow mechanism for the given reaction is shown below.
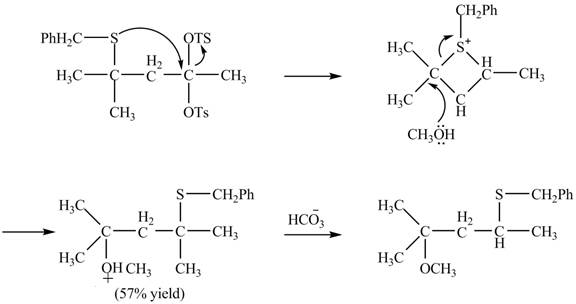
Figure 16
The curved arrow mechanism for given rearrangement is shown in Figure 16.
(i)
Interpretation:
The curved arrow mechanism for the given reaction is to be shown.
Concept Introduction:
The vicinal
Answer to Problem 11.77AP
The curved arrow mechanism of the given reaction is shown below.
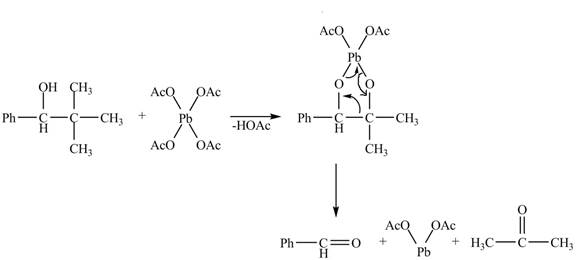
Explanation of Solution
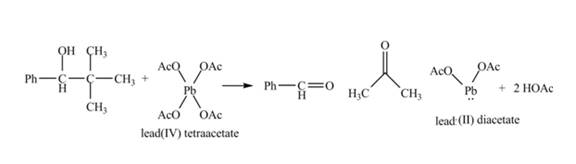
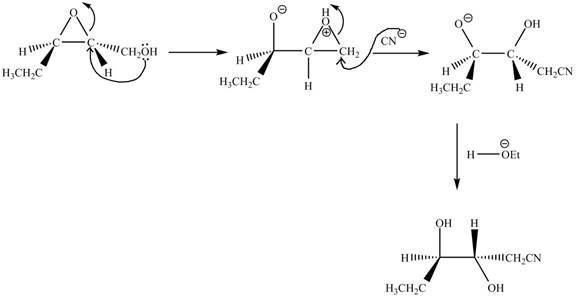
Figure 17
The given compound is a vicinal diol. It will react with the lead tetraacetate to form five membered ring intermediate. The intermediate formed rearranges to form acetone, benzaldehyde and lead diacetate. The curved arrow mechanism for the given reaction is shown below.
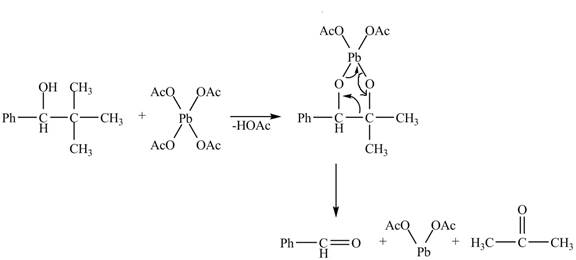
Figure 18
The curved arrow mechanism for given reaction is shown in Figure 18.
Want to see more full solutions like this?
Chapter 11 Solutions
Loose-leaf Version For Organic Chemistry
- Write a curved arrow mechanism for the following transformation:arrow_forwardA. Arrange the following radicals in order of decreasing rate of bromination. Justify your answer. B. Trehalose and isomaltose are both dimers of glucose. However, they have considerablydifferent reactivities. Concisely explain why these differences are observed. -Isomaltose is a reducing sugar while trehalose is not.-Trehalose is very resistant to acid hydrolysis while isomaltose can be acid-hydrolyzed withease.arrow_forwardComplete the curved arrow notation and include the structure of the missing organic intermediate for the following steps of a nucleophilic aromatic substitution via an addition–elimination mechanism.arrow_forward
- (Please give clear handwritten answer) Provide an arrow pushing mechanism for the following transformation. Classify all pericyclic reactions.arrow_forwardDraw a reasonable arrow -pushing mechanism that explains formation of the given products.arrow_forwardPredict the major organic product of the following reaction for a and or barrow_forward
- Give a clear handwritten answer with explanation...give the missing reagents for this reaction..complete the following reactionarrow_forwardGive the major organic product of the following reaction for a) or b)arrow_forwardGive a clear explanation handwritten answer...give the mechanism for Friedel acylation with curved arrow..arrow_forward
- Draw the arrow pushing mechanism for the reaction of camphor to isoborneol. For each step on the mechanism give one DETAILED sentence on what is occurring and why. Examples: "Loss of water to form a tertiary carbocation", "nucleophilic attack of ____ to increase acidity of ____", "methyl shift of ____ to form ____", etc. Thank you!arrow_forwardplease help with this question. thank you. The following sequence, beginning with a cyclic hemiacetal (compound A), was part of a recently reported enantiospecific synthesis of a powerful sex pheromone (currently used in pest management) of the mealybug Pseudococcus viburni: Draw the structures of compound B and C. Provide a plausible mechanism to explain the transformation from compound C into compound D. Identify the reagents you would need to convert compound D into compound F (in just two steps). Also identify the structure of compound E.arrow_forwardCompound F may be synthesised by the method attached Draw the isomer of compound B and explain which one would be the major product and why.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
