
Concept explainers
(a)
Interpretation:
The line structure of
Concept Introduction:
Line structure: Line structure is a simplest representation of a hydrocarbon. In line structure, the chain of carbon atoms is shown as a zigzag line. The end of each short line in the zigzag represents a carbon atom. Since the carbon nearly always has a valence of four in organic compounds, it is not necessary to show the hydrogen atoms.
Class of hydrocarbons:
(a)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of triple bond in the compound shows that it belongs to alkyne.
(b)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of single bond in the compound shows that it belongs to alkane.
(c)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of double bond in the compound shows that it belongs to alkene.
(d)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 11A.1E
The line structure of
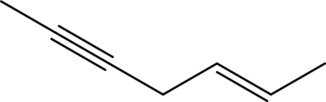
In
Explanation of Solution
The given condensed formula is
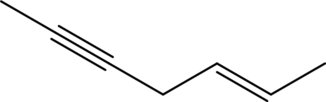
In
(e)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(e)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of double bonds in the compound shows that it belongs to alkene.
Want to see more full solutions like this?
Chapter 11 Solutions
CHEMICAL PRINCIPLES PKG W/SAPLING
- There are 11 structures (ignoring stereoisomerism) with the formula C4H8O that have no carbon branches. Draw the structures and identify the functional groups in each.arrow_forwardDraw and name the eight cycloalkane structures of formula C6H12 that do not show geometric isomerism.arrow_forwardDraw the structures of all monobromo derivatives of pentane, C5H11Br, which contain a 4-carbon chain.arrow_forward
- Draw the structures of the 3 isomers of C8H18 that contain 3 methyl branches on the main chain, 2 of which are on the same carbon.arrow_forwardDraw the structures of the 2 isomers of C8H18 that contain 2 methyl branches on the same carbon of the main chain.arrow_forwardhow many alkanes of the formula c6h14 have quaternary carbon atom?arrow_forward
- which of butane, propane, methane, ethane and methylcyclohexane contains only primary carbons in their structure?arrow_forwardGive the molecular formula of a hydrocarbon containingfive carbon atoms that is (a) an alkane, (b) a cycloalkane,(c) an alkene, (d) an alkyne.arrow_forwardwrite the structure formulas of alkanes with molecular formula C6H14, which with chlorine give: a) three monochlorinated isomers? b) five monochlorinated isomers c) only two monochlorinated isomersarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





